What is the difference between physical and chemical changes?
The essential difference between physical and chemical changes lies in whether the substance’s composition is altered or remains the same during the change. Understanding the differences between physical and chemical changes helps to identify how substances interact in everyday processes and industrial applications.
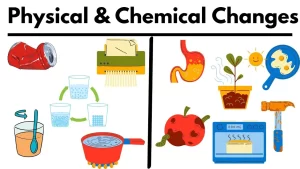
Physical and Chemical changes in the matter
The changes that may occur to the matter are the physical changes and the chemical changes.
The melting of a candle is a physical change, while the burning of a candle is a chemical change, The freezing of any liquid matter as water is a physical change.
The evaporation of the water to form the water vapour and the condensation of the water vapour into the water drops are physical changes, The boiling of water and the melting of any solid matter are physical changes.
Physical changes
A physical change is a change in the shape of the matter without any change in its structure (properties).
Examples of the physical changes
- When you grind the chalk or the sugar into the powder.
- The change of the water (The matter) from one state to another state (ice cycle).
- When any solid matter melts as the wax, the chocolate, and the ice.
- When you dissolve the table salt or the sugar in the water.
- When you cut the paper into small pieces and when you make paper recycle.
- The malleability, the ductility, and the bending of the elements.
Chemical changes
The chemical changes in the matter produce a new matter such as in burning, rusting, cooking, and Film processing, The chemical change is a change in the structure of the substance producing a new substance or new substances with different properties.
Examples of chemical changes
- When you burn any matter such as (wood, sugar, paper, or a candle).
- when the iron rusts, the shape and the structure of the cleaning iron change as it is exposed to the wet air.
- When you add sodium bicarbonate to vinegar.
- When you add the yeast to the pastry (dough).
- In the production of yoghurt from milk.
- In the fermentation of the fruits or the sugar.
- In the digestion of the food.
Chemical changes vs. Physical changes
1. Definition
Physical Change is a change in which the form or appearance of a substance changes, but its chemical composition remains the same, No new substances are created. Example: Melting ice into water – it changes from solid to liquid, but it’s still H₂O.
Chemical Change is a change in which a substance undergoes a transformation that alters its chemical structure, resulting in the formation of one or more new substances with different properties. Example: Burning wood – the wood chemically reacts with oxygen to produce ash, carbon dioxide, and water vapor.
2. Composition
Physical Change: The molecular structure of the substance remains unchanged, meaning no new chemical bonds are formed or broken. Example: Crushing a can – it’s still aluminum, only its shape is altered.
Chemical Change: In chemical Changes, The chemical composition of the substance changes, and new chemical bonds are formed or existing ones are broken. Example: Rusting of iron – iron reacts with oxygen to form iron oxide, a new substance.
3. Reversibility
Physical changes are often reversible. The substance can be returned to its original form by physical means (like freezing water back into ice). Example: When you dissolve sugar in the water – the sugar can be recovered by evaporating the water.
Chemical changes are irreversible under normal conditions, When a chemical change occurs, it is not easy to reverse it without another chemical reaction. Example: When you cook an egg – the proteins in the egg undergo a chemical change when heated, and you cannot uncook the egg.
4. Energy Change
Physical Change involves changes in energy, such as heat or light, but the energy changes are usually smaller compared to chemical changes and do not alter the chemical bonds. Example: Boiling water – heat energy is required to change the state from liquid to gas, but the molecular structure of water remains unchanged.
Chemical changes involve energy changes, either releasing energy (exothermic) or absorbing energy (endothermic), due to the breaking and formation of bonds. Example: a large amount of energy is released as heat and light in the combustion of gasoline.
5. Change in Properties
Physical Change: Only physical properties (such as shape, size, state, and appearance) change, but the chemical properties remain the same. Example: Shredding paper – it’s still paper but in smaller pieces.
Chemical Change: The product of the chemical reaction often has properties different from the original substances as both physical and chemical properties of the substance change. Example: Vinegar reacting with baking soda – the substances combine to produce carbon dioxwateride gas and water, with properties different from vinegar or baking soda alone.
6. Examples
Physical Changes:
- Melting ice into water.
- Freezing water into ice.
- Boiling water to steam.
- Cutting paper.
- Stretching a rubber band.
Chemical Changes:
- Burning wood.
- Rusting of iron.
- Cooking food.
- Digesting food.
- Mixing baking soda and vinegar (producing carbon dioxide gas).
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Heat Changes accompanying Chemical changes and Hess’s law of constant heat summation
Heat changes accompanying physical changes and Explanation of the source of the heat of solution
Atoms components, Rutherford and Bohr’s Atomic Models
Balanced chemical equations, Law of conservation of matter (mass) and Law of constant ratios