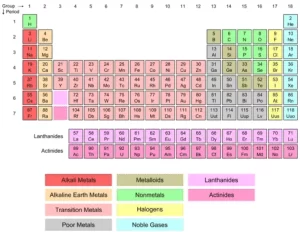
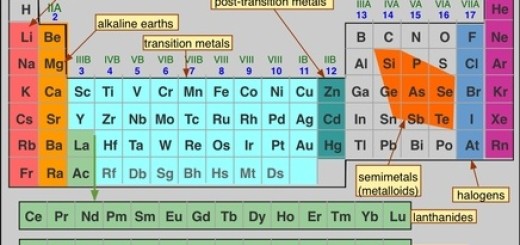
Lanthanides and Actinides (Inner Transition Metals) in the modern periodic table
Lanthanides and actinides are located below the modern periodic table, They consist of two rows, They are known as the f-block elements because they have valence electrons in the f-shell, Lanthanides elements can be found naturally on Earth, and only one element of them is radioactive.
Lanthanides and actinides
Lanthanides are 14 elements with atomic numbers 57 (Lanthanum, La) to 71 (Lutetium, Lu), and Actinides are 14 elements with atomic numbers from 89 (Actinium, Ac) to 103 (Lawrencium, Lr).
Lanthanides and Actinides belong to the periods 6 and 7, respectively and between groups 3 and 4, and they are set off below the main portion of the table, All of the actinides are radioactive and some of them are not found in nature. They are two series of elements that belong to the inner transition metals in the periodic table. They are placed separately at the bottom of the table to maintain its structure.
Lanthanides are an important group of elements, Most of the lanthanides are formed when uranium and plutonium undergo nuclear reactions, Lanthanides are the elements produced as the 4f sublevel is filled with electrons and the actinides are formed while filling the 5f sublevel.
Actinides are the elements found in the second period of the f-block, and these elements fill up the 5 f sublevel, Lanthanides are the rare earth elements found in the first period of the f– block, and these elements fill up the 4 f sublevel.
1. Lanthanides (Rare Earth Elements)
The atomic Numbers: 57 (Lanthanum) to 71 (Lutetium), Location: Period 6, f-block
General Properties:
- Silvery-white metals.
- High melting and boiling points.
- Typically trivalent (+3 oxidation state).
- Exhibit similar chemical properties due to the lanthanide contraction.
- Used in electronics, magnets, lasers, and catalysts.
Elements:
- Lanthanum (La)
- Cerium (Ce)
- Praseodymium (Pr)
- Neodymium (Nd)
- Promethium (Pm) (radioactive, rarest)
- Samarium (Sm)
- Europium (Eu)
- Gadolinium (Gd)
- Terbium (Tb)
- Dysprosium (Dy)
- Holmium (Ho)
- Erbium (Er)
- Thulium (Tm)
- Ytterbium (Yb)
- Lutetium (Lu)
2. Actinides
The atomic Numbers: 89 (Actinium) to 103 (Lawrencium), Location: Period 7, f-block
General Properties:
- All are radioactive.
- Can have multiple oxidation states (+3 to +6).
- Some are highly reactive and toxic.
- Used in nuclear reactors, weapons, and medical applications.
Elements:
- Actinium (Ac)
- Thorium (Th)
- Protactinium (Pa)
- Uranium (U) (used in nuclear fuel)
- Neptunium (Np)
- Plutonium (Pu) (used in nuclear weapons & reactors)
- Americium (Am) (used in smoke detectors)
- Curium (Cm)
- Berkelium (Bk)
- Californium (Cf) (used as a neutron source)
- Einsteinium (Es)
- Fermium (Fm)
- Mendelevium (Md)
- Nobelium (No)
- Lawrencium (Lr)
Uses of lanthanides and actinides
- Oxides of lanthanides and actinides are used in the glass industry for polishing and making colored glass for goggles and television screens.
- Due to the paramagnetic and ferromagnetic nature of the lanthanides and actinides, they are used in the magnetic and electronic devices.
- Mixed oxides of the lanthanides are used in petroleum cracking, and ceric sulphate is well-known as an oxidizing agent which is used in the volumetric analysis.
- Lanthanides and actinides do not find any use in their pure state, So they are used in the production of alloys of steel to improve the strength and workability of the steel.
Importance of Lanthanides and Actinides
Lanthanides and actinides play a crucial role in modern technology, medicine, energy, and research. Their unique properties make them valuable in various applications.
1. Importance of Lanthanides
Lanthanides, also known as rare earth elements, are widely used in high-tech industries due to their magnetic, optical, and catalytic properties.
Major Applications:
Electronics & Communication:
- Used in smartphones, laptops, and televisions (e.g., phosphors in screens).
- Neodymium (Nd) and Dysprosium (Dy) are used in high-strength magnets for electric motors and speakers.
Renewable Energy:
- Neodymium and Samarium are essential for making powerful magnets used in wind turbines.
- Lanthanum (La) and Cerium (Ce) improve battery performance in hybrid and electric vehicles (EVs).
Optics & Lasers:
- Ytterbium (Yb) and Erbium (Er) are used in fiber-optic communication systems.
- Holmium (Ho) and Thulium (Tm) are used in laser surgery.
Catalysts & Chemical Processing:
- Cerium (Ce) is used in catalytic converters in vehicles to reduce pollution.
- Lanthanum (La) is used in refining petroleum and manufacturing optical glass.
Medical Applications:
Gadolinium (Gd) is used in MRI contrast agents for better imaging.
2. Importance of Actinides
Actinides, especially uranium and plutonium, are critical for nuclear energy and medical advancements.
Major Applications:
Nuclear Energy & Power Generation:
- Uranium-235 (U-235) is used as a fuel in nuclear reactors to generate electricity.
- Plutonium-239 (Pu-239) is used in nuclear power and weapons.
- Thorium (Th) is being researched as an alternative nuclear fuel.
Medical Uses:
- Americium-241 (Am-241) is used in smoke detectors and as a radiation source in medical imaging.
- Californium-252 (Cf-252) is used in cancer treatment and neutron radiography.
Scientific Research:
- Actinides are used in space exploration for radioisotope thermoelectric generators (RTGs), providing power for spacecraft like Voyager and Curiosity Rover.
- They help in studying nuclear reactions and particle physics.
Military & Defense:
- Plutonium (Pu) is a key component in nuclear weapons.
- Uranium (U) is used in armor-piercing ammunition and naval reactors.
Lanthanides are crucial for technology, renewable energy, and medical imaging, while actinides are vital for nuclear energy, space exploration, and medical treatments. Their applications make them essential elements for scientific progress and daily life.