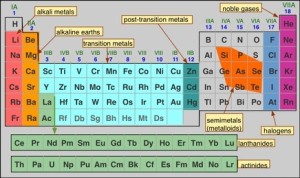
Graduation of metallic and nonmetallic property in the modern periodic table
Modern periodic table
In the modern periodic table, each period starts with the strong metal (in group 1 (1 A), where the metallic property decreases by increasing the atomic number, till we reach the metalloids.
Then the nonmetallic property appears, and increases by increasing the atomic number till we reach the strongest nonmetal (in group 17 (7 A) and ends in the inert gas (group zero).
By increasing the atomic number (within a period), the metallic property decreases, while the nonmetallic property increases.
In metallic groups, the metallic property increases gradually as we go from top to bottom as in group (1 A) (because the atomic size increases).
When you look within group (1 A) in the modern periodic table, you will notice the graduation of the metallic property, Cesium (Cs) is the most metallic element in the group (1 A), and lithium (Li) is the least metallic element in the group (1 A).
In nonmetallic groups, the nonmetallic property decreases gradually as we go from top to bottom as in group (7 A) (due to the decrease of the electronegativity).
When you look within group 7 A (17) in the modern periodic table, you will notice the graduation of nonmetallic property, Flourine (F) is the strongest nonmetal element in group (7 A). and Iodine (I) is the least nonmetal element in the group (7 A).
When you look at the modern periodic table, you notice that there is a direct relationship between the atomic size of an element and its metallic property.
There is a direct relationship between the electronegativity of an element and its nonmetallic property.
You can download Science online application on google play from this link: Science online Apps on Google play
Metallic & nonmetallic property, Acidic & basic property in the periodic table
Atoms components, Rutherford and Bohr’s Atomic Models
Modern periodic table and classification of Elements
Radius property, Ionization potential, Electron affinity & Electronegativity
Graduation of the properties of the elements in the modern periodic table