General properties of alkali metals in the modern periodic table
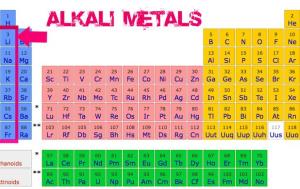
The alkali metals are located on the left side of the modern periodic table in the group (1) or (1 A). They are the first group of s-block, The alkali metals are mono-valent elements as they have only one electron in their outermost energy levels.
Alkali metals
The alkali metals are all metals that have one electron in the outer shell, they are very reactive, they are the most reactive metals where they are located in group 1, the alkali metals are good conductors of heat and electricity.
Most alkali metals have low density. Lithium (Li). Sodium (Na) and potassium (K) elements float on the water surface as their densities are smaller than the water density. Rubidium (Rb) and cesium (Cs) elements sink in the water as their densities are greater than the water density.
The alkali metals tend to lose their valency (outermost) electrons during the chemical reactions forming positive ions, Each of them carries one positive charge.
The elements of the alkali metals react with the water forming the alkaline solutions, So, They named the alkali metals.
The alkali metals react to lose electrons, These metals are very reactive with reactivity increasing down the group, They are active elements, so, they are kept under the surface of kerosene or paraffin oil.
Lithium is not kept under the surface of kerosene as it floats on its surface and burns at once so, it is kept in paraffin oil, Sodium and potassium are kept under the surface of kerosene to prevent them from the reaction with the moist air as they are active metals.
If you would like to know why Cesium is the most active metal, Look at the modern periodic table, You will know that the chemical activity of the alkali metals increases as the atomic size increases, therefore, Cesium (Cs) is considered the most active metal (in the periodic table) as it has the largest atomic size.
You can download Science online application on google Play from this link: Science online Apps on Google Play
Elements of s-block, Properties of the first group elements 1A (Alkali metals) in the periodic table
Alkali metals compounds properties and uses (Sodium hydroxide & Sodium carbonate)
Metallic & nonmetallic property, Acidic & basic properties in the periodic table
Elements of s-block, Properties of the first group elements 1A (Alkali metals) in the periodic table