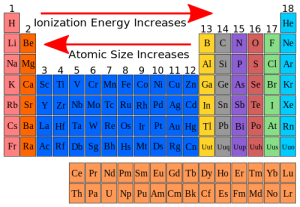
Atomic size of the elements in the modern periodic table
The atomic radius is used as a measure for the atomic size of the atom, and its measuring unit is picometre (Pm), The picometre is part from million of million part of metre.
There is a graduation of the atomic size of the elements in the periodic table where the atomic size for the elements of the same period is inversely proportional to the atomic number.
The atomic size decreases in the periods by increasing the atomic number (from the left to the right), due to the increase of the attraction force between the positive nucleus, and the outermost electrons.
The atomic size for the elements of the same group is directly proportional to the atomic number, The atomic size increases in the groups, by increasing the atomic number (from up to down), due to the increase of the number of the energy levels occupied by the electrons.
The nucleus is positively charged as it contains the protons, and the neutral neutrons, In the modern periodic table, You notice that the largest atom of the elements in size is the cesium atom, and the smallest one is fluorine.
You can download Science online application on google play from this link: Science online Apps on Google play
Graduation of the properties of the elements in the modern periodic table
The graduation of the metallic and nonmetallic property in the modern periodic table
Atoms components, Rutherford and Bohr’s Atomic Models
Evolution concept of the atomic structure, Atomic theory & Properties of cathode rays
Modern periodic table and classification of Elements
Chemical combination, Types of bonds (Chemical bonds & Physical bonds)


Can i have the lesson completly
You can read these articles
The graduation of the electronegativity of the elements in the periodic table
The lanthanides and actinides in the modern periodic table
The properties and uses of some elements in the modern periodic table
The description of the modern periodic table
The general properties of halogens in the modern periodic table
The general properties of the alkali metals in the modern periodic table
Atoms components , Rutherford and Bohr’s Atomic Models