Valency of elements, How can you calculate the valency of each element?
The valency is the number of electrons that an atom gains, loses, or even shares during a chemical reaction. The valency is the number of electrons that an atom needs to gain or lose to achieve noble gas electronic configuration.
The valency
Valency is a measure of the ability of an atom to bond with other atoms, typically determined by the number of electrons an atom needs to gain, lose, or share to achieve a stable electron configuration (usually a full outer shell, or octet rule).
In the chemical reaction, the atoms try to reach the stable state by losing their outermost electrons (as in the case of metals) or by sharing a number of electrons with other atoms (as in the case of nonmetals).
The valency of the elements or the ions to write the chemical formula of a compound. Chlorine has a valency of 1 as it needs to gain 1 electron to achieve a noble gas electronic configuration.
The atoms try to reach the stable state by gaining a number of electrons to complete the outer shell by (8) electrons (as in the case of nonmetals).
The outermost electrons of an atom determine its valency, the valency of an element measures its ability to combine with the other elements, The valency is determined by the number of electrons in the outer shell of each atom of an element.
The valency of some elements in the modern periodic table
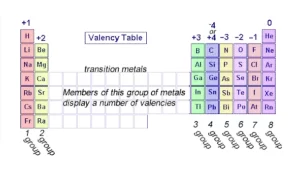
The elements in group (1 A) in the modern periodic table have one valent electron in their outer shell; they are very active, they are mono-valent elements as they have only one electron in their outermost energy levels.
The valency of the alkaline earth metals in group (2 A) is divalent; they are divalent elements as they have two electrons in their outermost energy levels.
The elements of group (7 A) are mono-valent elements as their outermost energy levels have (7) electrons, The elements in group (18) in the modern periodic table are the noble gases that have a valence of zero as the valence shell is saturated with 8 electrons.
To determine the valency of the ionic molecule phosphorus tetraoxide (PO4, four atoms of oxygen, and one atom of phosphorus) you should multiply the total valency of the four oxygen atoms (valency 2) and subtract that from the valency of the phosphorus atom (valency 5). That reveals the valency of (PO4) is 3.
The valency of an element is always a whole number, and some elements exhibit more than one valency, they have more than one valency such as sulphur (S) is divalent, tetravalent, and hexavalent.
Nitrogen (N) is trivalent and pentavalent, while phosphorus (P) is trivalent and pentavalent. The valency of copper I is monovalent, while the valency of copper II is divalent.
The valency of Iron [II] sulfate or ferrous sulfate is divalent as in FeSO4, and the valency of iron is two, while the valency of Iron [III] sulfate or ferric sulfate is trivalent as in Fe2(SO4)3, the valency of iron is three.
How to find valency
1. Determine the Atomic Number
The atomic number gives the number of protons in an atom and, for neutral atoms, equals the number of electrons. This is essential because valency is related to the number of electrons in the outer shell.
2. Write the Electron Configuration
Distribute the electrons in the shells according to the 2n² rule, where:
- The first shell (K-shell) can hold up to 2 electrons.
- The second shell (L-shell) can hold up to 8 electrons.
- The third shell (M-shell) can hold up to 18 electrons, but for most elements, it follows the octet rule (up to 8 electrons in the outer shell).
For example, the electron configuration of carbon (atomic number 6) is 2, 4, meaning there are 2 electrons in the first shell and 4 in the second shell.
3. Identify the Number of Electrons in the Outermost Shell
The number of electrons in the outermost shell determines how an atom will react to achieve a full outer shell. These electrons are called valence electrons.
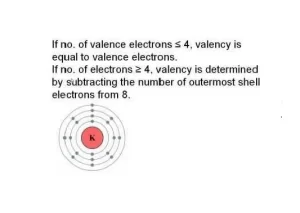
- If the number of valence electrons is less than or equal to 4, the element tends to lose electrons to achieve stability.
- If the number of valence electrons is greater than 4, the element tends to gain or share electrons.
4. Calculate Valency
For elements with 1 to 4 valence electrons (e.g., hydrogen, lithium, carbon), the valency is equal to the number of valence electrons.
For elements with more than 4 valence electrons (e.g., oxygen, chlorine), the valency is equal to 8−valence electrons8−valence electrons, since they tend to gain electrons to fill their outer shell.
Valency of Common Elements
- Hydrogen (H): Atomic number 1, electron configuration 1 → 1 valence electron → Valency = 1
- Helium (He): Atomic number 2, electron configuration 2 → Full outer shell → Valency = 0 (inert gas)
- Carbon (C): Atomic number 6, electron configuration 2, 4 → 4 valence electrons → Valency = 4
- Oxygen (O): Atomic number 8, electron configuration 2, 6 → 6 valence electrons → Valency = 8 – 6 = 2
- Sodium (Na) has an atomic number of 11, electron configuration is 2, 8, 1 → 1 valence electron → Valency = 1
- Chlorine (Cl) has an atomic number of 17, electron configuration is 2, 8, 7 → 7 valence electrons → Valency = 8 – 7 = 1
Transition metals can have more than one valency due to their d-electrons. For example, iron can have a valency of 2 or 3. Inert (noble) gases like helium, neon, and argon have full outer electron shells and thus have a valency of 0 since they do not typically react or form bonds.
General method for calculating the valency of elements
- For elements with 1-4 valence electrons, Valency = Number of valence electrons.
- For elements with 5-7 valence electrons, Valency = 8 – Number of valence electrons.
You can follow Science Online on YouTube from this link: Science online
What is Valency? Valencies of some metallic, nonmetallic elements & atomic groups
Modern periodic table and classification of Elements
The quantum numbers and principles of distributing electrons
Metallic & nonmetallic properties, Acidic & basic properties in the periodic table
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept

It helped me a lot i can now understand what valency is
Thank you very much for your comment
it helped me a lot…………you are doing very good for students keep it up…………..thanks a lot!!!
You are welcome
Thank you very much for your comment
thank you so much.. i can clearly understand about valency now…
You are welcome
Thank you very much for your comment
This helped to understand the valency.good efforts.
Thank you very much for your comment
its help very much
thanking you heartfully
You are welcome
Thank you very much for your comment
P+O2 =P2O3O
Number of atoms of reactants must equal number of atoms of products
4P + 3O2 = 2P2O3
How valency and reaction done.
4P + 3O2 = 2P2O3
Number of atoms of reactants must equal number of atoms of products
Can you explain me haber process
You can read this article about Preparation of ammonia gas in industry ( Haber-Bosch’s method )
The most famous elements in 5A group ( Nitrogen properties , Preparation & compounds )
baie dankie
This article really helped me …thanks
Cover more and more topics under your articles ..
You are welcome