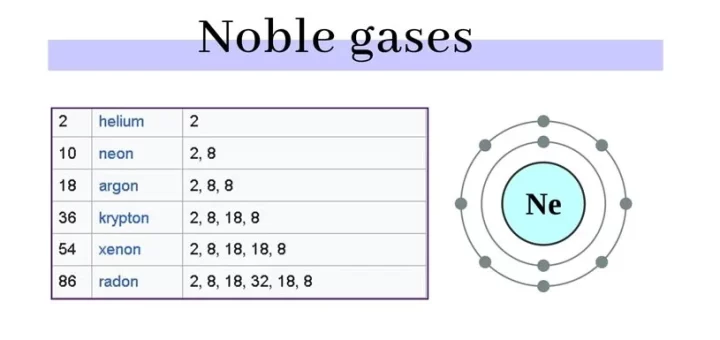
Properties of Noble (inert) gases, Features and Applications of Inert gases
Inert gases are the elements in which the outermost electron shells are completely filled with electrons, so they don’t participate in any chemical combination in ordinary conditions.