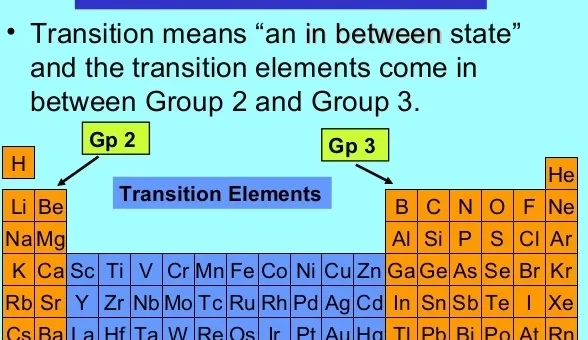
General properties of the first transition elements in the modern periodic table
Most of the transition elements are attracted to the external magnetic fields because the spin of unpaired electrons in the sublevel (d) produces a magnetic field that makes the atom attracted to the external...