Celiac disease (CD) cause, stages, symptoms, treatment and What is a CD test?
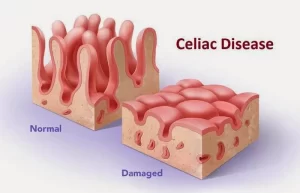
Celiac disease (CD) is characterized by mucosal inflammation, villous atrophy, and crypt hyperplasia, which occurs upon exposure to dietary gluten in genetically susceptible individuals and which demonstrates improvement after withdrawal of gluten from the diet. It is one of the most common causes of chronic malabsorption, due to loss of absorptive surface area, and reduction of digestive enzymes.
Celiac disease (CD)
CD is an autoimmune disorder triggered by the consumption of gluten, a protein found in wheat, barley, and rye, When people with celiac disease eat gluten, their immune system reacts by 2 attacking the small intestine. This damage can make it difficult for the body to absorb nutrients from food.
Epidemiology
- Celiac disease is the most common genetically related food intolerance, worldwide.
- Estimated 3 million in USA and 3 million in Europe.
- On the rise in Africa, Asia and the Middle East.
- Slightly more in females.
- Bimodal age distribution: First 8-12 months, Second 3rd -4th decade.
- Mean age 8.4 years.
- In developing countries, the prevalence rates are overlapping European figures: 0.53% in Egypt, 0.79% in Libya, 0.6% in Tunisia, 0.88% in Iran, 0.6% in Turkey
- Increased incidence in patients with type I Diabetes, Down and Turner’s syndrome.
Pathophysiology
Celiac disease is an autoimmune disease and the enzyme tissue transglutaminase (TTG) has been discovered to be the autoantigen against which the abnormal immune response is directed. Gluten is the single major environmental factor that triggers the celiac disease, which has a narrow and highly specific association with class II haplotypes of HLA DQ2 and to a lesser extent D08.
Clinical presentation
- Celiac disease is not only a disease of S.B. but it’s a multi-organ Al disease.
- It is grossly underdiagnosed, and it has significant complications.
- Classification: It may be:
- Symptomatic: Classical or Atypical.
- Asymptomatic: Silent or Latent (Potential celiac disease).
Symptomatic
1- Classical
The classic definition of celiac disease includes the following three features: villous atrophy; symptoms of malabsorption and resolution of the mucosal lesions and symptoms upon withdrawal of gluten-containing foods, usually within a few weeks to months.
2- Atypical
Patients with atypical disease exhibit only minor gastrointestinal complaints. They can display extraintestinal manifestations.
However, there is a shift from patients presenting with classic celiac disease to more patients with atypical symptoms of an asymptomatic presentation.
Asymptomatic
- Silent: No S&S, +ve Ab, Abnormal bx.
- Latent (CD in remission): No S&S, +ve Ab, normal bx.
Two variants of what has been called latent celiac disease have been identified:
- Celiac disease was present before, usually in childhood; the patient recovered completely with a GFD, remaining “silent” even when a normal diet was reintroduced. Approximately 20 percent of such patients continue to have latent disease into adulthood, while the others redevelop variable degrees of villous atrophy. Latency may be transient and thus regular follow-up of such patients is warranted.
- A normal mucosa was diagnosed on an earlier occasion while ingesting a normal diet, but CD developed late.
Gastrointestinal Manifestation (Typical)
- Diarrhea -45-85% of patients.
- Adult patients with undiagnosed celiac disease rarely present with profuse diarrhea and severe metabolic disturbances (celiac crisis).
- Flatulence – 28% of patients.
- Weight loss – 45% of patients/ in infants and young children with untreated a celiac sprue, failure to thrive and growth retardation are common.
- Weakness and fatigue – 78-80% of patients; usually related to general poor nutrition.
- Severe abdominal pain – 34-64% of patients.
- Anorexia, nausea, vomiting, constipation.
- Angular stomatitis, angular cheilosis.
Extraintestinal manifestations (Atypical)
- Skin: Dermatitis herpetiform.
- Musculoskeletal: (Metabolic bone disorder). Dental enamel hypoplasia, osteopenia, osteoporosis, arthropathy (Nonerosive polyarticular symmetrical, large joints, muscle cramps, and tetany.
- Hepatobiliary: Hepatic steatosis (elevated transaminase levels), Hepatitis, Cholangitis.
- Hematological: Hyposplenism (Prophylactic pneumococcal vaccination has been suggested), iron-deficient anemia resistant to oral iron
- Cardiac: Carditis, Cardiomyopathy.
- Neurological: Peripheral neuropathy, Ataxia, Fits.
- Psychiatric: Depression, Anxiety (One-third of patients).
- Reproductive: Female sub-infertility, recurrent abortions, low birth weight babies.
- Renal: Glomerular IgA deposition is common, occurring in as many as one-third of patients. The great majority of affected patients have no clinical manifestations of renal disease.
- Pulmonary: Idiopathic pulmonary hemosiderosis also known as Lane-Hamilton syndrome.
Dermatitis Herpetiformis
Symmetric intensely pruritic vesicles, crusts and erosions distributed over the extensor areas of the elbows, knees, buttocks, shoulders, and I scalp, with a tendency to the grouping of individual lesions.
Diagnosis of CD
- The clinical manifest is compatible with the disease.
- Serological markers.
- SB bx findings.
- Clinical response to a gluten-free diet.
- All testing should be performed while patients are on a gluten-rich diet. No single test can confidently establish the diagnosis of CD in every individual. As a result, the most important initial step in diagnosis is recognizing the many clinical features associated with the disease.
Antibody testing
- IgA Anti-tissue Transglutamine Antibody (IgA TTG) is the best first test, although biopsies are needed for confirmation. IgA- TTG: 95% sensitivity and 95% specificity in untreated CD.
- Endomysial IgA.
- Reticulin IGA.
- IgA Anti-Deaminated Gliadin antibody (IgA DGP).
- Nonspecific patients with CD have a high incidence of total IgA deficiency (3-5%) So total IgA should be determined beforehand. If IgA is deficient, IgG Transaminase can be measured.
- Electrolytes.
- Hemoglobin and CBC.
- Stool Examination.
- Genetic testing.
- Bone densitometry.
HLA typing
- The HLA-DQ2 and DQ8 genotypes are abnormal in virtually all patients with celiac disease. The HLADQ2 test picks up 95%, and the HLA-DQ8 another 5% of patients. The negative predictive value for both tests is higher than 99%.
- The problem with this test- and the reason we don’t test everybody with this genetic test — is that 25%-30% of the white population of the United States will test positive, but they don’t have celiac disease.
Upper Endoscopy 6 duodenal biopsies
- Considered the standard criterion to establish the diagnosis.
- Endoscopic findings: Mucosal fold scalloping, Reduced mucosal folds, Mosaic pattern Crossby’s capsule: For jejenal biopsies.
Imaging
- Dilatation of the small intestine.
- Coarsening or obliteration of the mucosal pattern.
- Fragmentation or flocculation of the barium in the gut lumen.
Histology
- Mucosa is only involved.
- Villi are atrophic or absent with a decreased villous-to-crypt ratio.
- Crypts are hyperplastic.
- Proliferation of the plasma cells and lymphocytes.
FIGURE
- Duodenal bulb, mucosal nodularity/mosaic pattern;
- Total villous atrophy; water-immersion technique with magnification;
- The second portion of the duodenum; water-immersion technique, mucosal fissures, and scalloping of folds.
- Areas of total villous atrophy interspersed with areas of partial villous atrophy (patchy villous atrophy).
Causes of small intestinal villous atrophy other than celiac disease
- Small intestinal bacterial overgrowth.
- Crohn disease.
- Cow’s milk or soy protein intolerance (children).
- Eosinophilic gastroenteritis.
- Giardiasis.
- Intestinal lymphoma.
- Peptic duodenitis.
- Post-gastroenteritis.
- Tropical sprue.
- Zollinger-Ellison syndrome.
- Common variable immunodeficiency.
- Autoimmune enteropathy.
- Other immunodeficiency states (usually apparent clinically, eg, AIDS enteropathy, hypogammaglobulinemic sprue).
- Medications (eg, olmesartan).
- Whipple disease.
- Malnutrition.
- Intestinal tuberculosis.
- Graft-versus-host disease.
Histology criteria
The established celiac lesion shows typical morphological features including:
- Increased numbers of intraepithelial lymphocytes (IELs).
- Decreased enterocyte height.
- Villous atrophy.
- Crypt hyperplasia.
The severity of histologic changes in the small bowel does not necessarily correlate with the severity of clinical manifestations. Although there is a gradient of decreasing severity from the proximal to the distal small intestine, correlating with the higher proximal concentration of dietary gluten. Quantitative histology (villous height, crypt depth, density of IELs per 100 enterocytes) provides the most sensitive and accurate method to monitor disease activity over time.
Modified Marsh classification
Upper endoscopy with at least 6 duodenal biopsies is considered the criterion standard to help establish a diagnosis of celiac disease. Marsh’s classification is the histological classification system of CD most widespread and used worldwide both for diagnostic purposes and for histological reevaluation after the institution of a gluten-free diet. Duodenal biopsies can be graded into the following 5 stages:
- Stage 0: Normal.
- stage 1: Increased percentage of intraepithelial lymphocytes (>30%).
- Stage 2: Increased presence of inflammatory cells and crypt cell proliferation with preserved villous architecture.
- Stage 3: Mild (A), moderate (B), and subtotal to total (C) villous atrophy.
- Stage 4: Total mucosal hypoplasia.
Complications
- Increased risk of malignancy: Adenocarcinoma of the oropharynx and esophagus, pancreas, small and large bowel, hepatobiliary tract.
- T-cell lymphoma.
- Non-Hodgkin lymphoma.
Treatment
- Gluten-free diet.
- Because lactase deficiency is commonly associated avoid milk products.
- Vitamins, calcium, and iron, help in the start phase of therapy to enhance improvement.
- Patients with latent CD are currently not advised to be on a GFD but should continue to be monitored and rebiopsied if symptoms develop, However, such patients must be evaluated with multiple intestinal biopsies since histologic abnormalities can be patchy.
- Recently immunosuppressed Steroids in refractory celiac.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Gastroesophageal reflux disease (GERD) cause, treatment and How to treat eosinophilic esophagitis?
Pharynx function, anatomy, location, muscles, structure, and Esophagus parts
Tongue function, anatomy and structure, Types of lingual papillae and Types of cells in taste bud
Mouth Cavity divisions, anatomy, function, muscles, Contents of Soft palate and Hard palate
Temporal and infratemporal fossae contents, Muscles of mastication and Otic ganglion
Stomach parts, function, curvatures, orifices, peritoneal connections and Venous drainage of Stomach