Acid-base balance importance, Defense against changes in Hydrogen ion and Threats of pH
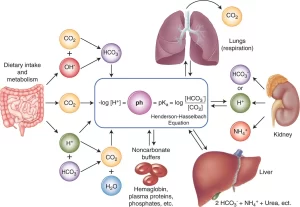
The normal pH of arterial plasma = 7.4 ± 0.02, the pH of venous plasma and of interstitial fluids= 7.38 ± 0.02 because of the extra quantities of carbon dioxide that form carbonic acid in these fluids, Acidosis is present when arterial pH falls below 7.38 and alkalosis when the arterial pH rises above 7.42.
Hydrogen ion balance
Hydrogen ions are the most reactive cations in body fluids, and they interact with negatively charged ions of other molecules, such as those of body proteins, Interactions of hydrogen ions with negatively charged functional groups of proteins can lead to marked changes in protein structural conformations with resulting alterations in the behavior of the proteins.
Alterations in the structural conformations and charges of protein enzymes will obviously affect their activities, with resulting alterations in the functions of body tissues, The absorption, and efficacy of drugs administered by the physician may also be affected by the pH.
PH = log 1/(H+ conc) = – log H+ concentration
Extreme changes in the hydrogen ion concentration of the body can result in loss of organ system function and structural integrity; under acute conditions, arterial pH above approximately 7.80 or below 6.9 is not compatible with life, Control of pH is necessary because of the marked effects of pH changes on protein conformation, enzymatic reactions, and central nervous system function.
Threats to PH
The major threats to the pH of the body fluids are acids formed in metabolic processes, These metabolic acids can be divided into three categories.
Volatile acids: Carbon dioxide
CO₂, a major end product in the oxidation of carbohydrates, fats, and amino acids, can be regarded as an acid because of its ability to react with water to form carbonic acid (H2 CO3) which in turn can dissociate to form H+ and HCO3:
CO₂ + H₂O ⇔ H₂CO3 ⇔ H+ + HCO3−
CO₂ adds approximately 10.000 mmol. of H+ to the body fluids per day.
Fixed acids
Sulphuric acid and phosphoric acid
Sulphuric acid is an end product of the oxidation of sulphur-containing amino acids, methionine, and cysteine. However, phosphoric acid is formed in the metabolism of phospholipids, nucleic acids, phosphoproteins, and phosphogly cerides, In contrast to CO₂, sulphuric acid and phosphoric acid are non-volatile, They result in the formation of 50-100 mmol. of H+ /day.
Organic acids
Lactic acid, acetoacetic acid, and B-OH-butyric acid are formed during the metabolism of carbohydrates and fats. Normally, these acids are further oxidized to CO₂ and H₂O and therefore do not directly affect the pH of the body fluids. However, in certain abnormal circumstances, these organic acids may accumulate causing acidosis.
- In hypovolemic and other forms of circulatory shock, lactic acid levels may accumulate due to inadequate perfusion of tissues resulting in an increase of anaerobic glycolysis (lactic acidosis).
- In uncontrolled diabetes mellitus, acetoacetic acid and B-OH-butyric acid may accumulate due to increased lipid metabolism (diabetic ketoacidosis).
Defense against changes in H+ concentration
- Acid-base buffer systems in body fluids.
- The respiratory system.
- The kidneys.
The buffer systems can act within a fraction of a second, The respiratory system takes 1-12 minutes to act, The kidneys though the most powerful require several hours to several days to readjust the H+ concentration.
Acid-base buffers
An acid-base buffer is a solution containing a combination of two or more chemical compounds that prevent marked changes in hydrogen ion concentration when either an acid or a base is added to the solution. An acid is a substance that can add hydrogen ions to a solution. A base is a substance that combines with hydrogen ions in a solution and will thereby remove them.
The bicarbonate buffer system
A typical bicarbonate buffer system consists of a mixture of carbonic acid (H₂CO3) and sodium bicarbonate (NaHCO3) in the same solution. Carbonic acid is a very weak acid. When a strong acid, such as hydrochloric acid, is added to a bicarbonate buffer, the following reaction takes place.
HCI + NaHCO3 ===> H₂CO3 + NaCl
From this equation, it can be seen that the strong hydrochloric acid is converted into very weak carbonic acid, Therefore, the HCI lowers the pH of the solution only slightly. On the other hand, if a strong base, such as sodium hydroxide, is added to the same bicarbonate buffer solution, the following reaction takes place:
NaOH + H₂CO3 ===> NaHCO3 + H₂O
The net result is the change of the strong base NaOH for the very weak base NaHCO3, Fortunately, this buffer is present in all the body fluids, both extracellular and intracellular, and it plays an important role in maintaining normal acid-base balance.
The phosphate buffer system
The phosphate buffer system is composed of a mixture of acidic phosphate H₂PO4− (Na di-hydrogen phosphate: NaH₂ PO4) and alkaline HPO4−-(Na mono-hydrogen phosphate: Na₂H PO4). The phosphate buffer system is more effective in ICF than in ECF, since:
- The total concentration of phosphate is much greater in ICF than ECF.
- The intracellular pH is somewhat lower than the extracellular pH and therefore is closer to the pka of the phosphate buffer, (pka = 6.8).
The phosphate buffer system is also effective in buffering the tubular fluid in the kidneys because:
- Phosphate becomes greatly concentrated in the tubular fluid due to there absorption of water in excess of phosphate.
- The pH of the tubular fluid is more acidic than the pH of ECF and therefore is closer to the pH of the phosphate buffer.
Protein buffer system
- The plasma and the intracellular proteins.
- Hemoglobin: Hemoglobin is a major buffer in the blood. Once hemoglobin becomes deoxygenated in the capillaries, it is more able to bind the H+ ions formed when CO₂ enters capillary blood from tissues. Thus, hemoglobin helps to minimize the fall in pH caused by the loading of CO₂ into capillary blood.
Respiratory regulation of acid-base balance
The lungs regulate the pH by regulating the partial pressure of CO₂ in arterial blood. When the blood carbon dioxide concentration increases, the PCO₂ in both the interstitial fluid of the medulla and the cerebrospinal fluid also increases, The CO₂ immediately reacts with water to form carbonic acid, and carbonic acid dissociates into H+ ions and HCO3−.
Thus H+ concentration increases in the chemoreceptors of the medulla oblongata and stimulates alveolar ventilation by direct action, Moreover, the increase in arterial PCO₂ stimulates alveolar ventilation indirectly through the chemoreceptors in aortic and carotid bodies.
When the blood CO₂ concentration increases in the medulla oblongata, more H+ appear in the chemoreceptors reaction than when the blood hydrogen ion concentration increases, as H+ ions do not easily cross either the blood-barrier or the blood cerebro-spinal fluid barrier.
The decrease in arterial pH that occurs independently of an increase of arterial PCO₂ stimulates the chemoreceptors in the carotid bodies. However, a decrease in PCO₂ and H+ concentration will decrease ventilation, Respiratory regulation of the pH is effective but almost never complete. Respiratory control returns the hydrogen ion concentration 50-70% to normal.
Renal regulation of blood pH
The kidneys regulate the blood pH by secretion of hydrogen ions and by regulating the concentration of HCO3− in plasma.
1. Tubular secretion of hydrogen ions
The epithelial cells of the proximal, distal tubules and collecting ducts all secrete hydrogen ions into the tubular fluid.
2. Regulation of plasma HCO3
The kidneys regulate the plasma HCO3− by two mechanisms:
- Reabsorption of the filtered bicarbonate.
- Generation of new bicarbonate.
Reabsorption of the filtered bicarbonate
- Step 1: Blood CO₂ is in equilibrium with CO₂ in the tubular cells. In the cell, CO₂ reacts with H₂O in the presence of carbonic anhydrase to form carbonic acid, Carbonic acid dissociates into hydrogen ions and bicarbonate ions.
- Step 2: The hydrogen ion is secreted from the brush border of the tubular cell into the tubular fluid by sodium-hydrogen counter-transport (to maintain electroneutrality inside the tubular cells).
- Step 3: In the tubular fluid, hydrogen ions are buffered with the filtered bicarbonate forming carbonic acid, This carbonic acid is dissociated to form CO₂ and water, The CO₂ molecule moves across the tubular epithelium.
- Step 4: The bicarbonate ion generated in step 1 passes passively across the tubular cell membrane to the peritubular fluid accompanied by the reabsorbed sodium ion, In this way, filtered sodium bicarbonate is returned to the blood.
Generation of new bicarbonate
The kidneys generate HCO3− to replace the HCO3− lost in buffering the various strong acids formed in the body. About 100 mmol of H+ is added to the body fluids each day from the fixed acids produced during metabolism. The body’s buffer systems, particularly the bicarbonate buffer, provide the most immediate defense against this H+. For example, sulphuric acid produced during the metabolism of sulphur-containing amino acids is buffered by HCO3−.
H₂SO4 + 2NaHCO3 → Na2SO4 + 2H₂CO3 → Na2SO4 + 2 CO2 + 2H₂O
CO₂ formed in this reaction is eliminated by the lungs. The net result of the reaction is the loss of two HCO3 ions from the body, which must be replaced by the kidneys.
The main buffer in the tubular fluid is the bicarbonate buffer (HCO3−). However, since the reaction between H+ and HCO3−. results in the reabsorption of filtered HCO3−, excess H+ must be excreted by combining with non-bicarbonate buffers in the tubular fluid, the most important of which are ammonia and phosphate.
Ammonia
Ammonia (NH3) is a highly effective buffer for secreted H+, NH3 is synthesized and its concentration in the epithelial cells increases, it diffuses out of the cells, Although it can diffuse into either the tubular lumen or the peritubular capillaries, its diffusion into the lumen (I.e. NH+ secretion) is favored because the secreted NH3 immediately reacts with previously secreted H+ to form NH4−.
Ammonium ion, due to its electrical charge, combines with a chloride ion forming ammonium chloride which is very weakly acidic, Sodium-ion is exchanged with hydrogen and is absorbed into the blood as a new sodium bicarbonate, From this reaction 2 NH3 molecules are formed and 2 new HCO3− are generated and enter the blood.
Sufficient NH3 is therefore secreted to react with virtually all the secreted H+ that cannot be buffered by the low-capacity phosphate buffer system, allowing the 50-100 mmol of excess H+ that must be excreted per day to be excreted with a minimum fall in the tubular fluid pH. The formation of NH3 in the epithelial cells increases in acidosis and decreases in alkalosis.
Phosphate
In the distal nephron, the phosphate concentration in the tubular fluid is significantly increased due to the reabsorption of water in excess of phosphate. Sodium mono-hydrogen phosphate (Na2HPO4) changes to the more acidic sodium di-hydrogen phosphate (Na₂HPO4). One sodium ion is replaced by one hydrogen ion secreted by the tubular cells.
Sodium ion is reabsorbed with the generated bicarbonate into the blood. Thus, for each hydrogen ion combined with phosphate, one molecule of new sodium bicarbonate is added to the blood. In a normal man, the amount of phosphate delivered to the distal nephron allows only 12-40 mmol of H+ to be excreted as Na2HPO4 each day. However, the amount of available phosphate remains relatively constant even in acidotic conditions.
Variations in urinary pH
Urinary pH ranged from 4.5-8; pH 4.5 is the limiting pH, which is the minimal pH of urine below which no H+ secretion can occur.
You can subscribe to science online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Acid-base disturbances, metabolic acidosis causes, Effects of acidosis and alkalosis on the body
Physical properties of urine, Tests to evaluate kidney function and Normal constituents of urine
Urinary bladder structure, function, Control of micturition by Brain and Voluntary micturition
Urine formation, Factors affecting Glomerular filtration rate, Tubular reabsorption and secretion
Histological structure of kidneys, Uriniferous tubules and Types of nephrons
Functions of Kidneys, Role of Kidney in glucose homeostasis, Lipid and protein metabolism