Transition elements and Economic importance of elements of the first transition series
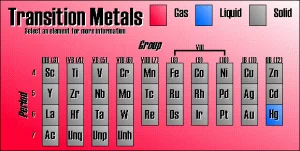
The transition elements occupy the middle of the periodic table between (s) and (p) blocks, Including more than 60 elements (more than half the number of known elements), These elements can be classified into main transition elements and inner transition elements.
Main transition elements
Main transition elements (elements of d-block) contain ten vertical columns because in the main transition elements, the electrons occupy the d-sublevel in sequence since the d-sublevel can take up to ten electrons.
Columns start from the first column which contains all elements that end with nS2, (n−1)d1 to the last one which ends with nS2, (n−1) d10 in which sublevel is filled in sequence, Columns from left to right of the periodic table start with the groups (3) IIIB, (4) IVB, (5) VB, (6)VIB, (7) VIIB, (8) VIII, (1) IB, (2) IIB.
Group (8) VIII contains three vertical columns (8), (9), (10) which differ from the other transition elements of (B) in their properties in which these elements are similar horizontally more the elements below in the same column, Main transition elements can be classified to four horizontal series which are:
The first transition series
It includes the elements in which the sublevel (3d) is filled successively, It is found in the fourth period after calcium, It consists of ten elements from scandium 21Sc (4S2, 3d¹) and ends with zinc 30Zn (4S2, 3d10), Although the elements of the first transition series represent 7% only from the weight of the earth’s crust, they have a great economic importance.
The second transition series
It includes the elements in which the sublevel (4d) is filled successively, It is found in the fifth period, It consists of ten elements starts with yttrium 39Y (5S2, 4d¹) and ended with cadmium 48Cd (5S2, 4d10).
The third transition series
It includes the elements in which the sublevel (5d) is filled successively, It is found in the sixth period, it consists of ten elements start with lanthanum 57La (6S2, 5d¹) and ended with mercury 80Hg (6S2, 4f, 5d10).
The fourth transition series
It includes the elements in which the sublevel (6d) is filled successively, They are found in the seventh period.
The economic importance of elements of the first transition series
Scandium (21Sc)
It is found in very small amounts spread over a large area in the earth’s crust, Alloys of (Sc + Al) are used in the manufacture of Mig fighter jets because adding a small amount of scandium to aluminum, it gives a light and very hard alloy, Scandium is added to mercury vapour lamps to produce light with high quality looks like sunlight, so it is used in TV photography at night.
Titanium (22Ti)
It is a strong element of rigidity as steal, but it is less denser than steel, Alloys of Titanium with aluminum are used in the manufacture of aircraft and space shuttle industry because it maintains its durability at high temperatures while the hardness of aluminum decreases.
Titanium is used in dental implants and artificial joints because it is an inert substance, so the body does not eject it and does not cause any type of poisoning, One of the most common compounds is Titanium dioxide (Ti O2) which is used in sun protection cosmetics because minute nanoparticles prevent effect of the Ultraviolet radiation on the skin.
Vanadium (23V)
Vanadium is used in the manufacture of car springs because when we add a small amount of it to the steel (ferrovandium), a high-hardness alloy is formed and has a great ability to resist corrosion.
Vanadium pentaoxide (V2O5) is used in the manufacture of dyes used in the ceramics and glass industry, also it is used as a catalyst and manufacture of strong magnetic conductors, It is used in the manufacturing of benzoic acid and sulphuric acid by contact method.
2SO2 + O2 → 3SO3
SO3 + H2O → H2SO4
Chromium (24Cr)
Chromium is a chemically active metal but it resists the effect of the atmospheric air due to the formation of a larger metal oxide on its surface which forms a nonporous layer of metal oxide that prevents the continued interaction with oxygen of air, the size of the formed oxide molecules is larger than the size of atoms of the element, chromium is used in metal plating and leather tanning, Chromium III oxide Cr2O3 is used in the manufacture of dyes, Potassium dichromate (K2Cr2O7) is used as oxidizing agent.
Manganese (25Mn)
Manganese is not used as a pure metal because it is a brittle metal so, it is always used in the form of alloys or compounds, Ferromanganese alloy is used in railway tracks because it is harder than steel, Aluminum and manganese alloys are used in the manufacture of soft drinks vessels (Cans) because they resist corrosion.
Manganese dioxide MnO2 acts as a strong oxidizing agent and it is used in dry cell battery, potassium permanganate KMnO4 is used in industry as an oxidizing agent and antiseptic substance, Manganese II sulphate MnSO2 is used as a fungicide and potassium permanganate solution is used in sterilization process because it is used as antiseptic.
Iron (26Fe)
Iron is used in the manufacture of reinforced concrete, electricity pylons, knives, guns, cannons, pipes and surgical tools, Also it is used as a catalyst in the manufacture of ammonia by Haper-Bosch method and the conversion of water gas (mixture of hydrogen and carbon monoxide) to a liquid fuel by Fischer-Tropsch method, Iron is widely used in many industries because Iron is the most common element on Earth by percentage weight.
Cobalt (27Co)
Cobalt is similar to iron in that both of them can be magnetized, so they are used in the manufacture of magnets, Cobalt is used in the manufacture of modern dry batteries that are used in modern cars, Cobalt has twelve radioactive isotopes, Cobalt 60 is the most important one because it produces gamma rays which have high penetrating power, so, it is used in:
- Food preservation process.
- It is used for the detection of the quality of industrial products by detecting internal defects such as cracks and welding connections.
- It is used in medicine, It is used for the diagnosis and treatment of tumors.
Nickel (28Ni)
Nickel is used in the industry of nickel-cadmium batteries which can be recharged, Nickel forms with steel alloys which are hard and resist rust and the effect of acids, Nickel-chromium alloys are used in heating coils and electric furnaces because they resist corrosion at high temperatures.
Nickel is used for painting the other metals to protect them from oxidation and rust and gives these metals a beautiful appearance, Divided nickel is used as a catalyst like in the hydrogenation processes of oil.
Copper (29Cu)
Copper is the first discovered metal, Copper–tin alloys are known as bronze alloys, Copper is a good conductor of electricity, so, it is used in the electric cables and coins industry, Fehling solution (one of the copper compounds) is used to detect glucose in which its blue colour changes to orange, Almost all of copper salts and solutions are blue, Copper II sulphate (CuSO4) is used as insecticide and fungicide in the purification of water.
Zinc (30Zn)
Zinc is used to galvanize other metals, such as iron, to prevent rusting, Galvanization is the process of applying a protective zinc coating to steel or iron, to prevent rusting, Zinc oxide ZnO is used in the manufacture of paints, rubber, and cosmetics, Zinc sulphide ZnS is used in the manufacture of illuminating paints and X-rays screens.
Properties of the first transition elements
These elements share similar properties, including:
- Metallic character: They are all metals with a shiny appearance.
- High melting and boiling points: They have strong metallic bonds, which contribute to their high melting and boiling points.
- The first transition series are good conductors of heat and electricity: They have free electrons that can move easily through the metal, allowing them to conduct heat and electricity.
- Variable oxidation states: They can form ions with different charges, depending on the number of electrons they lose.
- Colorful compounds: Many of their compounds are brightly colored due to the d-d electron transitions.
- Magnetic properties: Some of them are magnetic, such as iron, cobalt, and nickel.
The first transition series elements have a wide range of applications in industry, technology, and everyday life. For example, iron is used in steel, copper is used in electrical wiring, and titanium is used in aircraft and spacecraft.
Classification of elements in Long form periodic table, Ionization Energy and Oxidation numbers
First transition elements properties, electronic configuration & oxidation states