Properties of Strong nuclear forces, Source of nuclear binding energy & Quark Model
The nucleus occupies a small space from the volume of the atom, It contains a number of nucleons ( protons and neutrons), Protons are positively charged & neutrons do not have a change , But , did you ask yourself , How does the nucleus keep its stability in spite of the huge electric repulsive forces ( named coulomb electric force ) between the positive protons compared with the small attractive forces between the nucleons ?
That’s due to the presence of other forces working on the combining of these nucleons , These forces are called the strong nuclear forces .
Strong nuclear forces
The forces that bind the nucleons inside the nucleus , The forces that bind the nucleons are named by strong nuclear forces because they have the great effect on the nucleons inside the small nucleus .
Properties of the strong nuclear forces
It is a great force , It doesn’t depend on the essence ( type ) of nucleons , but it may be between : ( proton – proton ) , ( proton – neutron ) , ( neutron – neutron ) and it is a short-range force , These forces arise from the binding energy between the nucleus constituents , which is working in combining the nucleons together .
Source of nuclear binding energy
Many accurate measurements proved that : The mass of binded nucleons ( actual mass of nucleus ) is less than the mass of the free nucleons ( theoretical mass of nucleus ) .
Mass defect = theoretical mass – actual mass
Where theoretical mass = [ no. of protons × the mass of the proton ] + [ no. of neutrons × the mass of neutron ]
The loss in mass ( mass defect ) is converted into energy to bind the constituents of the helium nucleus , where is named nuclear binding energy , Nuclear binding energy is the amount of energy that is equivalent to the decrease ( loss ) in the mass of the nucleus constituents .
The nuclear binding energy ( BE ) can be calculated from this relation :
Nuclear binding energy ( BE ) = mass defect × 931
Nuclear binding energy ( Me V ) , mass defect ( u )
The value in which each nucleon contributes in the binding energy of the nucleus is called the Binding energy per nucleon , Nuclear stability increases by increasing the value of the binding energy per nucleons ( BE / A ) .
Binding energy per nucleon = BE / A
BE is the binding energy , A is the mass number ( no. of nucleons ) , Actual mass of nucleus is less than the theoretical mass because a part of the mass of nucleus constituents converted into energy to bind the constituents together in the nucleus .
Nuclear stability
The term of nuclear stability is used to describe the probability of the nucleus of element’s atom to decay with time , So , elements can be classified according to their nuclear stability into stable elements and unstable elements .
Stable elements
Stable element is the element in which its atom’s nucleus remain stable by passing time without any radioactivity .
Unstable elements
Unstable element is the element in which its atom’s nucleus decays by passing time as a result of radioactivity .
The ratio between the number of neutrons and protons ( N / Z ) determines the extent of the nuclear stability : When N = Z , This region is formed by nuclei of the stable element and is named as Belt of stability , Regarding the nuclear stability , there are two types of nuclei :
Atoms nuclei of the stable elements
The number of neutrons equals the number of protons , The ratio ( N / Z ) of their nucleons equals 1 , Such as light elements ( whose nucleons number is less than 38 ) .
The ( N / Z ) ratio increases gradually by increasing the atomic number till the ( N / Z ) ratio reaches to its maximum , which is 1.536 in the nucleus of lead isotope .
Atoms’ nuclei of the unstable elements :
At the left side of stability belt : The reason of the instability of atoms’ nuclei : no. of neutrons is larger than the stability level ( N / Z ratio is large ) .
How the unstable nuclei reach the stability state : By emitting beta particles ß− ( negative nucleus electron ) from the atom’s nucleus of the unstable element to transform one of the extra neutrons to proton and ( N / Z ) ratio approaches the stability belt .
At the right side of stability belt : The reason of the instability of atoms’ nuclei : No of protons is larger than the stability level ( N/ Z ratio is small ) .
How the unstable nuclei reach the stability state : By emitting positron ß+ ( positive nucleus electron ) from the atom’s nucleus of the unstable element to transform one of the extra protons into neutron and ( N / Z ) ratio approaches the stability belt .
Above the stability belt : The reason of the instability of atoms’ nuclei : No. of nucleons is larger than the stability level .
How the unstable nuclei reach the stability state : By emitting alpha α from the atom’s nucleus of an unstable element to decrease number of nucleons ( 2 protons , 2 neutrons ) to approach the stability belt .
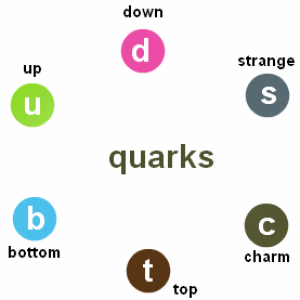
Quark Model
In 1964 , the scientist Murry Gell-Mann proved that the protons are formed from primary particles called quarks , Quark is a primary particle that can’t exist freely and all nucleons are formed from it .
There are six types of quarks .
Quarks with a charge + ( 2/3 ) e , Such as Top quark ( t ) , charm ( c ) quark , up ( u ) quark .
Quarks with a charge – ( 1/3 ) e , Such as bottom ( b ) quark , strange ( s ) quark , down ( d ) quark .
The composition of the proton ( p )
The proton consists of three quarks ( d , u , u ) , the positive electric charge ( Q ) of the proton can be calculated as follows :
Qp = d + u + u = + 1 e
The composition of the neutron ( n )
The neutron consists of three quarks ( u , d , d ) , the neutral electric charge ( Q ) of the neutron can be calculated as follows :
Qn = u + d + d = 0
Example : The composition of the quarks in the nucleus of helium atom
The nucleus of helium atom consists of : 2 protons ( each one is composed of combination between 1 d quark and 2 u quarks ), 2 neutrons ( each one is composed of combination between 1 u quark and 2 d quarks ) .
Radioactivity, Nuclear reactions (Natural transformation of elements) and Half-Life time