Organic chemistry, Properties of Organic and inorganic compounds
Organic chemistry is concerned with compounds containing carbon atoms which are joined together in a chain except for oxides of carbon, carbonate salt, and cyanides, In 1806, Berzelius classified the compounds into organic and inorganic compounds, He considered that organic compounds are obtained from living organisms, They are formed by vital force which is found in living cells.
Vital force theory
Vital force theory ( Berzelius) supposed that organic compounds are formed inside cells of living organisms by the effect of vital force, it can’t be prepared in the industry from other compounds, and it is impossible to synthesize them in the laboratory.
Organic compounds are the compounds that have been thought they are extracted from plants and animals only, Inorganic compounds are the compounds that are extracted from mineral sources in the earth.
Wohler Experiment (preparation of Urea)
Wohler destroyed the vital force theory, he performed an experiment that was considered to be the beginning of the end of Berzelius theory, Wohler was able to prepare Urea, which is an organic compound by heating an aqueous solution of two inorganic compounds (Ammonium chloride and silver cyanate solution).
Where ammonium cyanate is an inorganic compound while Urea is an organic compound that is considered a component of (the urine of mammals), Since that time, Scientists have tried to synthesize organic substances in the lab, where these compounds are used widely in all fields of our life.
NH4Cl (aq) + AgCNO (aq) → AgCl (s) + NH4CNO (aq)
NH4CNO(aq) → H2NCONH2 (s)
So, organic compounds can be prepared in industry, The failure of vital force theory to explain the formation of the organic compounds, because urea (an organic compounds) is prepared by heating an aqueous solution of ammonium chloride and silver cyanate (inorganic compounds).
Organic compounds
Not all compounds containing carbon atoms are considered organic compounds as carbon monoxide (CO), Carbon dioxide (CO2), Carbonates (CO3), bicarbonates (HCO3), Cyanides (CN), Cyanates (CNO) & carbides, They are not considered as organic compounds because their properties are different from that of organic compounds.
The abundance of organic compounds is due to the ability of carbon atom to bind with itself or with others by several methods by single, double or triple covalent bonds forming straight chains, branched and unbranched chains or heterocyclic or homocyclic compounds.
Homocyclic compounds are organic compounds in which all the corners of the ring have carbon atoms only, Heterocyclic compounds are organic compounds in which the corner of the ring has carbon atoms and other atoms of different elements.
A number of organic compounds is more than the number of inorganic compounds, The Number of organic compounds is more than 10 million, while the number of inorganic compounds is about ½ million, so the ratio between organic and inorganic compounds is approximately 20: 1, Inorganic chemistry concerned with the other 111 elements or more.
The organic materials become known according to their structure not according to their sources because most of the organic compounds which are prepared in laboratories are not formed in living organisms, The first organic compound that was prepared outside the living cells is urea
Organic compounds are used in drugs, detergents, dyes, plastics, fertilizers, and insecticides, They mainly contain carbon atoms, Most are insoluble in water but soluble in organic solvent such as benzene, Melting point is low, Boiling point is low, Most have characteristic odour, They are inflammable and produce CO2 & H2O.
The kind of bonds in the molecule is covalent bonds, The organic compounds don’t conduct electricity because they are non-electrolytic covalent compounds, The rate of the chemical reaction is slow because it takes place between non-ionized molecules, They can be polymerized and many organic compounds have isomerism.
Inorganic compounds
They may contain carbon in addition to other elements, Most of them are soluble in water, themelting point is high, Boiling point is high, Most are odourless, They are not inflammable and if it is inflammable, it produces other gases.
The kind of bonds in the molecule is ionic bonds, They are electrolytes, good conductors of electricity, The rate of chemical reaction is often fast because it takes place between the ions, They can’t be polymerized and they have no isomerism.
Molecular & structural formula of organic compounds
Molecular formula is the formula that indicates the number and kind of the atoms which form the chemical compound and doesn’t show the kind of linkage between the atoms in the molecule.
Structural formula is the formula that shows the type and the number of atoms in addition to the kind of linkage between the atoms by the covalent bonds, and also shows the valency of each element in the compound which is equal to the total number of bonds surrounding its atom.
The structural formula is preferred rather than the molecular formula to express an organic compound because the molecular formula indicates the number and the type of elements that form the chemical compounds only, but the structural formula indicates the type of linkage between the atoms.
The number of covalent bonds around the atom indicates its valency, Each single covalent bond represents one valency, Each element in the organic compounds has a specific and constant valency, where it is tetravalent in carbon atom (4), monovalent in hydrogen (1), divalent in oxygen (2) and trivalent in nitrogen (3).
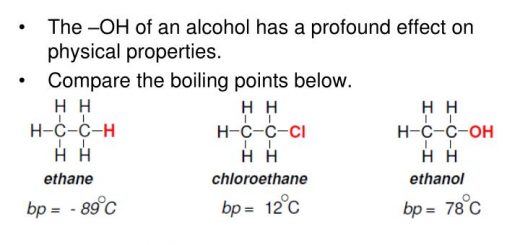
Ethyl alcohol and dimethyl ether have different properties although they have the same molecular formula (C2H6O) due to the difference in their structural formula, Ethyl alcohol replaces hydrogen of hydroxyl group, while dimethyl doesn’t react.
Properties of organic compounds
- Isomerism is the presence of many organic compounds could have the same molecular formula but they are different in their physical and chemical properties due to differences in bonds (differ in structural formula).
- Polymerization is the combination of a huge number of unsaturated simple molecules (monomers) which contain a small number of C-atoms, their number ranges from 100 to 1000000 to form a large molecule (polymer) which has the same empirical formula of the original compound and it has a large number of C atoms.
Hydrocarbons
The backbone of organic compound is composed mainly of carbon and hydrogen forming what is called hydrocarbons and remaining compounds are known as hydrocarbons derivatives, Hydrocarbons are organic compounds that have carbon and hydrogen only, They are Aliphatic [open chain (Acyclic) and cyclic saturated ] and Aromatic (cyclic unsaturated),
Open chain consists of :
- Saturated compounds are open-chain aliphatic hydrocarbon characterized by the presence of single bonds in the carbon chain.
- Unsaturated compounds are open chain aliphatic hydrocarbon characterized by the presence of double or triple bonds or more in the carbon chain.
Alkanes (Paraffins) are open chain aliphatic hydrocarbons in which the carbon atoms are combined together with a single bond of sigma type, their general formula is (CnH2n+2).
Alkenes (Olefins) are open chain aliphatic hydrocarbons, their general formula is (CnH2n), and between the carbon atoms of their molecules, one double bond or more are found.
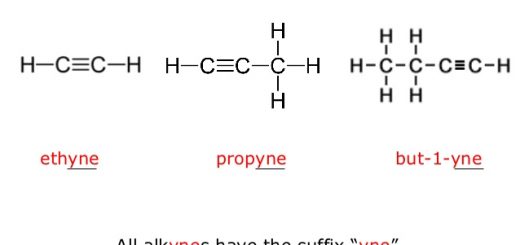
Alkynes (Acetylenes) are unsaturated open-chain hydrocarbons which contain at least one triple bond in the carbon chain, They form a homologous series, their general molecular formula is (CnH2n−2).
Alkanes are saturated compounds while alkenes are unsaturated ones, Alkanes are saturated because all carbon atoms combine together by single bonds, while Alkenes are unsaturated due to the presence of double bond or more between their carbon atoms.
Cyclopentane is a saturated compound, while aromatic benzene is an unsaturated one, Cyclopentane is a saturated compound because its carbon atoms combined together by single covalent bonds, while aromatic benzene is unsaturated due to the presence of 3 double bonds between its carbon atoms.
Detection of carbon & hydrogen in an organic compound
Steps:
- Put a small amount of an organic substance (textile – leather – paper – plastic) mixed with copper oxide (CuO) in a glass tube that resists heat.
- Heat the test tube strongly then pass the resulting gases over anhydrous white copper sulphate (CuSO4), then through lime water.
Observation:
- The white colour of anhydrous copper (II) sulphate turns into blue which indicates the absorption of (CuSO4) to water vapour which is formed from a combination of oxygen of copper (II) oxide with the hydrogen of the organic compound.
- Lime water turns turbid due to the evolution of carbon dioxide (CO2), which is formed from the combination of oxygen of copper (II) oxide with the carbon of the organic compound.
Conclusion: The organic compound contains carbon and hydrogen, Carbon is oxidized to CO2 which makes lime water milky, Hydrogen is oxidized to water which changes the white anhydrous CuSO4 into blue hydrated CuSO4.5H2O.
C + 2 CuO (s) → CO2 (g) ↑+ 2Cu (s)
2H + CuO (s) → H2O (v) + Cu (s)
Detection of carbon and hydrogen in an organic compound is carried out by heating it with copper oxide because carbon of the organic substance is oxidized by CuO into CO2 and hydrogen is oxidized by CuO into H2O which can be detected by lime water and anhydrous CuSO4 respectively.
Alkanes (Paraffins), Methane and Nomenclature of organic compounds