Laboratory Instruments use, How to prepare a solution? and How to dilute a concentrated solution
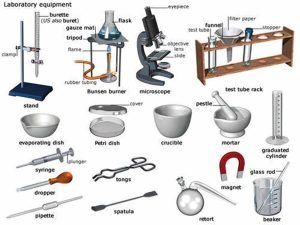
Laboratory equipment is used in the laboratory to carry out specific tasks, These tools are used by scientists, students, professors, and medical professionals, Some scientific lab equipment is used to weigh materials, mix and create solutions, and clean containers, Any experiment must be performed with care to prevent injury.
Laboratory Instruments
Instruments used for volume measurement: Instruments used for volume measurement depend on the accuracy and volumes needed, The smaller, the diameter of the instrument the more accurate it would be e.g. pipettes.
1. Pipettes
The pipette measures and delivers exact volumes of liquids.
- Glass graduated pipettes: Used to transfer small volumes of liquids (1ml-10ml).
- Automatic pipette: Most accurate of all, used to transfer micro-volumes of liquids e.g. (1μl-1000μl).
2. Graduated cylinders
The graduated cylinder is used to measure different volumes of liquids.
3. Volumetric flasks
The volumetric flask is used for the preparation of solutions with different concentrations and volumes.
Instruments for transfer, mixing, boiling, and storage of chemicals
- Test tubes: Used for performing chemical experiments and reactions in the lab, e.g. Holding liquid samples for heating, dissolution, centrifugation, and others.
- Reagents and chemical bottles: are used for the storage of different chemicals, reagents, and even strong corrosives. So, they should always carry an informative label.
- Graduated beakers: Used for a rough estimation of solution volume and for mixing different reagents.
- Conical (Erlenmeyer) flasks: More accurate than beakers in measuring the volume of solutions.
Glassware-Precision and Cost
Beaker
- The error of 5% of the total volume.
- Example for 1000 ml of solution, the error will be +/- 50 ml i.e range is 950 ml – 1050 ml.
- Imprecise; cheap.
Volumetric flask
- Error of 0.3%.
- Example for 1000 ml of solution, the error will be +/-0.30 ml. i.e. range is 999.70 ml-1000.30 mL.
- Precise; expensive.
Instruments used for handling chemicals
- A pipette pump is inserted into the end of the glass pipette to help the delivery of liquid without suction by mouth.
- Spatula resembles teaspoons, It resists corrosion, It is used to handle solid powdered chemicals.
- Droppers & plastic transfer Pasteur pipette: They are used to transfer small amounts or drops of liquid solutions.
Other lab tools and equipment
- Test tube Rack is for holding and organizing test tubes on the laboratory counter, Plastic racks may melt in contact with very hot test tubes.
- Test Tube Holder is useful for holding a test tube that is too hot to handle.
- Bunsen burner is used for the heating of nonvolatile liquids and solids.
- The lab water Bath offers precise temperature for lab reaction control.
- Funnels are used to aid in the transfer of liquid from one vessel to another.
- Balance is used to weigh chemicals.
- The centrifuge spins liquid samples at different high speeds to sediment different fractions.
Solutions
The solution is a mixture of two or more substances. e.g. if Nacl is added to water, the salt dissolves in water and a solution is formed, The solvent is the dissolving substance, water is an example, The solute is the substance being dissolved in the solvent. In the example, Nacl is the solute.
When the solvent has only a small quantity of solute dissolved in it, it is a dilute solution. When the solvent has a large amount of solute dissolved in it, it is a concentrated solution. These terms do not tell how much solute is present in the solvent.
How to prepare a solution?
The concentration of the solutions can be expressed as the following:
1. % (Percentage)
The term percent means per hundred, so when concentration is expressed as percent, tells how much solute is dissolved in one hundred parts (usually 100 ml) of the solution.
- % weight/volume (W/V) is the most commonly used.
- % V/V for liquids.
- % W/W for solids.
e.g. 1% Nacl = 1 g in 100 ml solution
2. Molar solution (M)
- It is the molecular weight in grams dissolved in a one-liter solution.
- e.g.:-Molar solution of NaOH = Molecular wt. of Na + O + H = 23 + 16 + 1 = 40
- ∴ molar solution of NaOH contains 40 gm/L.
- 2 molar solution of Nacl:
- Molecular wt. of Na + cl = 23 +35 = 58
- So, 2 molar solution of Nacl = 58 x 2 = 116 g/L
3. Normal solution (N)
- It is the equivalent weight in grams dissolved in one liter of solution.
- The equivalent wt = molecular wt./ valency
- If the valence is 1, molarity = normality
- e.g. 1 Normal solution of HCH is 1 Molar solution of HCI, But 1 Normal solution of H2SO4, 0.5 molar solution of H2SO4.
Molal solution (m):
These solutions contain 1 g mol (Molecular weight in g) per 1000 gm solvent. The advantage of this preparation lies in the fact that temperature never affects weight, consequently, the substance/solvent ratio will be fixed and osmotic pressure measurements will be more accurate.
How to dilute a concentrated solution?
Dilution
Sometimes chemists encounter strong solutions. Chemists can weaken the concentration of a solution by adding more solvent, a process known as dilution. After dilution, the quantity of solute remains the same, but the volume of solution has increased, thereby reducing the solution’s molarity.
Because the number of moles of solute does not change, we can apply a useful dilution equation, where M1 and V1 are the solution’s molarity and volume before dilution, and M2 and V2 are the solution’s molarity and volume after dilution.
M1 x V1 = M2 X V2
It is important to remember that the units for volume must remain consistent (V1 and V2 have the same volume units) in this equation. (Note: You may see the dilution equation represented as C1V1= C2V2, where C stands for “concentration.”). We can easily manipulate the dilution equation to determine any one of the missing four variables.
PH
Definition: pH is the potency of hydrogen ion (p = potency, H = hydrogen), or it is the measure of hydrogen ion concentration. Also, it is defined as the negative log of hydrogen ion concentration.
i.e. pH= − log [H+]
pOH equals − log [OH−]
The scale of pH is from 1-14:
When pH = 7 the solution is neutral as the number of [H+] = [OH−]
When pH is less than 7 (from 1-7), it is Acidic. when pH is more than 7 (from 7-14), it is basic.
Acid
It is a hydrogen ion donor or proton donor e.g.
Hcl→ H+ + cl−
Acids are either inorganic (as HCI) OR organic (such as acetic acid CH3COOH i.e. they possess a carboxyl group as an active group donating H+).
Base
Base is a hydrogen ion acceptor or proton acceptor.
Salt
It is a chemical compound formed, by the positive ion of a base and the negative ion of an acid.
H CI + NaOH → Na CI + H₂0
Also, the negative ion of the base (OH−) combines with the positive ion of the acid (H+) to produce water. Concentrations of acids and bases and strengths of acids & bases have H different meanings. Concentration refers to the amount of solute per liter of solution:
For example: 0.1 N HCL. 0.1 N CH3COOH. 0.I N H₂SO4 all have the same concentration. However, these acids have different strengths.
The strength of an acid is its ability to ionize, its readiness to donate protons to water. A strong acid is highly ionized. In solution, most of its hydrogen exists as the hydrogen ion (H+) Hcl. HNO3 and H2SO4, are strong acids because in solution they exist mainly as H+Cl− H+ NO3− and H+HSO3−, respectively. They are always strong acids regardless of their concentration.
A weak acid is only slightly ionized in solution. CH3COOH is a weak acid. In solution it exists mainly as CH3COOH, There is only a very small amount existing as CH3COO− H+. Carbonic acid is also a weak acid and in solution exists mainly as H2CO3.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download the Science Online application on Google Play from this link: Science Online Apps on Google Play
General Laboratory rules, Laboratory hazards, and Simple First Aid in the Laboratory
Classification of Acids according to its strength (degree of ionization), Its source & Basicity
Classifications of bases according to strength ( degree of ionization ) and molecular structure
Properties of Acids and Bases & Theories defining acids and bases
Types of compounds, Properties of Acids, Bases (alkalis), Oxides and Salts