Iron alloys, oxides, preparation, types, properties and importance
An alloy is usually formed from two metals, it is formed of two metals or more with a certain ratio, as iron alloys (ferromanganese, ferrochromium), and it may consist of metal and nonmetal with a certain ratio, as Iron and carbon, Transition elements are characterized by the formation of several alloys because the atomic radii of transition elements are relatively constant.
Alloys
An alloy is a substance that has the physical properties of metals. Metals are used industrially in alloys, not as pure metals, because alloys have new properties that differ from those of pure metals, such as:
- Alloys resist corrosion.
- Alloys can increase the magnetic and electric properties.
- Alloys can be shaped easily.
- Alloys are characterized by hardness.
Preparation of Alloys
- By melting: Alloys are usually prepared by melting the metals together and leaving them to cool gradually.
- By electrodeposition method: Alloys can be obtained by electrodeposition of two metals or more at the same time, such as electroplating Iron handles with brass (Cu + Zn) by its deposition from a solution of copper and zinc ions.
Types of Alloys
- Interstitial alloy
- Substitutional alloy
- Intermetallic alloy
Interstitial alloy
Alloys in which a metal whose atoms are smaller in size than those of the pure metal is introduced in the intermolecular spaces of the crystal lattice of the pure metal to increase its hardness and change the physical properties as malleability, ductility, melting point, electric conductivity, and magnetic properties, such as iron and carbon alloy (steel).
Pure iron as other metals is formed of the crystalline lattice of metal atoms in a closed-packed structure, On hammering the metal, a layer of atoms can slip over the other layers, In the state of introducing large atoms to form an Alloy, If the introduced atom is larger than those of pure metal, the structure of pure metal is deformed and the dislocation of the layer becomes more difficult, so, the alloy is hard than the pure metal.
In the state of introducing small atoms to form an Alloy, when the introduced atoms are smaller than the small spaces between the metal atoms, they will fit these spaces and will also lead to a change in the physical properties of the metal.
Properties of Interstitial Alloy
- Change malleability and ductility.
- Affects the melting point.
- Affects electric conductivity and magnetic properties.
- Affects the strength of the pure metal.
Substitution alloys
Alloys in which some atoms of the pure metal are replaced by atoms of other metal which has the same radius, crystal lattice, and chemical properties, such as Ferronickel alloy (Fe, Ni), Stainless steel Alloy (Fe, Cr), (Gold and copper) Alloy, and it takes place between two transition metals.
Conditions of substitutional alloy: They have the same diameter, crystalline structure, and chemical properties. Copper and Gold can form a substitutional alloy because they have the same diameter, crystalline structure, and chemical properties.
Intermetallic alloys (Compound Alloy)
Alloys formed as a result of a chemical combination between metals of different groups in the periodic table and the chemical formula of these compounds disobey the laws of valency, such as Aluminum-Nickel alloy (Ni3Al) which is called Duralumin and lead-gold alloy (Au2pb) and cementite (Fe3C), Duralumin is an alloy of aluminum and nickel, formed by the chemical combination (Ni3Al).
They are formed when the elements forming the alloy combine with each other chemically, forming chemical compounds which disobey the laws of valency, and they are solid. Chemical formula of these compounds disobey the laws of valency, and this alloy can’t be formed by metals of the same group.
Physical properties of iron
Pure iron has no industrial importance because it is relatively soft, It is quite reactive, with low hardness, malleable, ductile, with magnetic properties, It melts at 1538°C and its density is 7.87 gm/cm³, Physical properties of iron depend upon its purity and nature of its impurities, Steel and other iron alloys may be produced with specific properties suitable for many uses.
Chemical properties of iron
Iron differs from the elements before it in the first transition series, where it doesn’t have the maximum oxidation state corresponding to the removal of all electrons (8 electrons) from (4s and 3d), All the oxidation states above (+3) are not important, Iron has oxidation state (+2) which is corresponding to loss of two electrons from (4s) sublevel and oxidation state (+3) corresponding half-filled (3d5) sublevel (stable state).
Effect of air: Red-hot iron reacts with dry air or oxygen, producing iron oxide.
3Fe + 2O2 → Fe3O4
Effect of water vapour: Red-hot iron (> 500°C) reacts with water vapour, producing magnetic iron oxide and Hydrogen. Iron in this reaction is a reducing agent where red-hot iron (temperature > 500°C) reduces steam, as it removes oxygen, giving magnetic iron oxide.
3Fe + 4H2O → Fe3O4 + 4H2
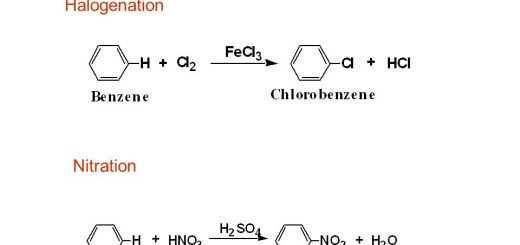
The effect with non-metals: Iron reacts with chlorine producing iron (III) chlorine and with sulphur producing iron (II) sulphide, sulphur is a weak oxidizing agent compared to chlorine as it has a smaller electronegativity, When iron reacts with chlorine, They produce iron III chlorine, not iron (II) chloride because chlorine is an oxidizing agent which oxidizes iron II chlorine into (III) chlorine.
2Fe + 3Cl2 → 2FeCl3
Fe + S → FeS
The effect with diluted acids: Iron dissolves in dilute mineral acids, producing iron (II) salts and Hydrogen, not iron (III) salts, which are reduced by the produced hydrogen.
Fe + H2So4 → FeSO4 + H2 ↑
Fe + 2HCl → FeCl2 + H2
When iron reacts with HCl produces iron II chloride, not iron III chloride, because the hydrogen produced reduces what is formed of iron III chloride to iron II chloride III. When iron reacts with diluted H2So4, Iron II sulphate is formed and not Iron III sulphate because the hydrogen produced reduces what is formed of iron III sulphate to Iron III sulphate.
Effect of concentrated acids: Iron reacts with hot concentrated sulphuric acid, producing iron (II) sulphate, iron (III) sulphate, sulphur dioxide, and water.
3Fe + 8H2SO4 → FeSO4 + Fe2 (SO4)3 + 4 SO2 + 8H2O
When Iron reacts with conc. H2SO4, both of iron II sulphate and iron III sulphate are formed because conc. Hot sulphuric acid is an oxidizing agent which oxidizes iron to (Fe3O4), while the acid is reduced to SO2 and H2O. At the same time, Fe3O4 reacts with the acid forming iron II sulphate, iron III sulphate, and water.
The passivity of iron is the formation of a thin nonporous layer of oxide, which protects the metal from further reaction with concentrated nitric acid. Concentrated nitric acid causes passivity of iron due to the formation of a thin layer of oxide, which protects the metal from further reaction. This layer can be removed by abrasion or by using dilute hydrochloric acid HCl.
Iron oxides
- Iron II oxide (FeO).
- Iron III oxide (Fe2O3).
- Magnetic iron oxide (Fe3O4).
Iron II oxide (FeO)
When heating Iron II Oxalate in the absence of air, iron II oxide is produced, not iron III oxide is formed because of the presence of carbon monoxide as a product of the reaction, which acts as a reducing agent and converts iron III oxide to iron II oxide. Fe2O3 is not formed due to CO is a reducing agent.
(COO)2Fe → FeO + CO + CO2
By reduction of higher oxides by hydrogen or carbon monoxide.
Fe2O3 + H2 → 2 FeO + H2O
Fe3O4 + H2 → 3 FeO + H2O
Properties of Iron II oxide
- It is a black powder, insoluble in water.
- Easily oxidized in hot air.
- It reacts with mineral acids, forming iron II salts and water.
4 FeO + O2 → 2 Fe2O3
FeO + H2SO4→ FeSO4 + H2O
Iron III oxide (Fe2O3)
Preparation: On adding an alkali to an aqueous solution of iron III salts, the (reddish brown) iron III hydroxide precipitates, and heating iron III hydroxide above 200°C, it converts to iron III oxides.
FeCl3 + 3NH4OH→ Fe(OH)3 + 3NH4Cl
2 Fe(OH)3 → Fe2O3 + 3 H2O
By heating iron II sulphate, iron III oxide is obtained.
2 FeSO4 → Fe2O3 + SO2 + SO3
Its existence: This oxide is present in nature in the form of hematite.
Properties
- It is insoluble in water.
- It is used as a red pigment in paints and in rouge.
- It reacts with hot conc. mineral acids to give iron III salts and water.
Fe2O3 + 3H2SO4 → Fe2 (SO4 )3 + 3H2O
The Black oxide (magnetic iron oxide) (Fe3O4)
Its existence: It can be considered as a mixed iron II and iron III oxides (FeO and Fe2O3) known naturally as magnetite.
Preparation:
- It is prepared by the action of hot air or steam on red-hot iron
- By reduction of iron III oxide.
3 Fe + 2O2 → Fe3O4
3 Fe + 4H2O → Fe3O4 + 4H2
3 Fe2O3 + CO → 2Fe3O4 + CO2
Properties
- It is a strong magnet.
- It reacts with hot concentrated acids to give iron II and iron III salts, proving that it is a mixed oxide.
Fe3O4 + 4H2SO4 → FeSO4 + Fe2 (SO4 )3 + 4H2O
It is oxidized to iron III oxide when it is heated in air.
2 Fe3O4 + ½ O2 → 3 Fe2 O3
On adding conc. hydrochloric acid to magnetite, a mixture of iron II chloride and iron III chloride is formed, as the magnetite consists of a mixture of iron II oxide and iron III oxide.
A piece of iron which is previously immersed in conc. Nitric acid does not react with copper sulphate solution because of the passivity phenomena, where a thin non-porous layer of oxide is formed on the iron surface and protects it from the reaction with copper sulphate.
It is preferable to use iron in the form of an alloy, not in the pure form, because pure iron is relatively soft with low hardness, malleable and ductile with magnetic properties, but alloys of iron are produced with specific properties suitable for many uses.
You can follow Science Online on YouTube from this link: Science online
Extraction of Iron from its ores, Iron dressing, Reduction & production