Heat content of a substance ( Molar enthalpy ) and Thermochemical Equation
Each substance stores an amount of energy inside it , This amount of energy called the internal energy , This amount of energy is the summation of three types of energy which are the stored chemical energy in the atom , The stored chemical energy in the molecule and the stored chemical energy between molecules .
The stored chemical energy in the atom is represented in the energy of the electrons in the energy levels and it is the summation of kinetic energy and potential energy of the electron in its energy level.
The stored chemical energy in the molecule is represented in the energy that is found in the chemical bonds between atoms ( ions ) with each other whether these bonds are ionic bonds or covalent bonds .
The stored chemical energy between molecule is represented in the intermolecular forces like Van der Waals forces ( It is considered as a potential energy ) and Hydrogen bonds ( These bonds depend on the nature of molecules and their polarity .
The sum of these energies in one mole of a substance is called the Molar Enthalpy ( H ) or The Heat Content of the Substance .
Heat content of a substance H ( Molar enthalpy )
It is the sum of the stored energies in one mole of a substance , The molar enthalpy of NO2 gas equals 33.58 kJ/mol means that the sum of the stored energies in 46 g ( 1 mol ) of NO2 gas equals 33.58 kJ .
The heat content differs from one substance to another because the molecules of different substances differ in the number and the type of atoms , the number and the type of bonds between their atoms .
It is not possible to measure the heat content for a certain substance practically , but we can measure the change that occurs to the heat content ( Δ H ) during the different changes that occur to the substance during the chemical reactions .
Heat content ( Δ H )
The difference between the sum of the heat content of the products and the sum of the heat content of the reacting substances .
The change in heat content = Heat content of the products – heat content of the reactants
Δ H = H products – H reactants
The change in heat content for different chemical reactions that carried out under the same standard conditions is called the change in standard heat content Δ H° .
These standard conditions are :
- Pressure that equals 1 atm ( normal atmospheric pressure ) .
- Temperature = 25° C ( room temperature ) .
- Concentration = 1 M ( molar concentration ) .
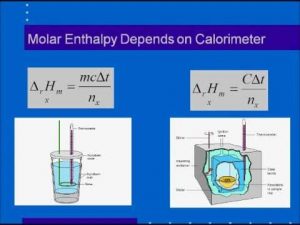
The change in the standard heat content Δ H° = the quantity of heat ( absorbed or liberated ) ( qp ) ÷ the number of moles of produced substance ( n )
Δ H° = qp / n
The heat content for the element = zero
Thermochemical Equation
A symbolic chemical equation that includes the heat change accompanying the chemical reaction and this heat change is represented in the equation as one of the reactants or products .
Conditions of thermochemical equation
- It must be balanced , If necessary , we can write the coefficients as fractions .
- The physical state of the reactants and the products must be written .
- Putting a positive or a negative charge beside ΔH : +ve ⇒ In case of absorbing heat ( endothermic ) , – ve ⇒ In case of liberating heat ( exothermic ) .
- When multiplying or dividing the coefficients of the two sides of the equation with a certain numerical coefficient , The same operation must be carried out on Δ H .
- The direction of the heat content can be inversed , where the sign of the heat content Δ H is inversed .
We can write the coefficients of balanced equation as fractions because the coefficients represent the number of moles of the reactants and products not the number of molecules or atoms .
The physical state of the reactants and the products must be mentioned in the thermochemical equation because the heat content changes with the change of the physical state of the substance .