Heat changes accompanying physical changes and Explanation of the source of the heat of solution
The calculation of the change in heat content is of a great importance where , the calculation of the change in heat content accompanying the burning of different fuels , helps us during designing the engines to know which type of fuel is more suitable .
The calculation of the change in heat content accompanying the burning of different materials , helps the firemen in identifying and choosing the most suitable method to put off the fire .
The change in the heat content differs according to the type of change ( physical or chemical ) .
Heat changes accompanying physical changes
Examples of heat changes accompanying physical changes are Standard heat of solution and Standard heat of dilution .
Standard heat of solution Δ H° sol
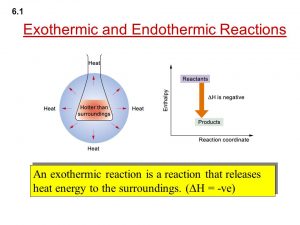
On dissolving a solid substance in a liquid , this process is accompanied by an increase or a decrease in the temperature of the resulted solution .
By dissolving Ammonium nitrate ( NH4NO3 ) in water , the temperature of the solution decreases due to absorbing an amount of energy .
The solution is called Endothermic solution .
NH4NO3 + H2O ( l ) → NH4+ ( aq ) + NO3− ( aq )
Δ H° sol = + 25.7 kJ / mol
On dissolving Sodium hydroxide ( NaOH ) in water , the temperature of the solution increases , due to releasing an amount of energy .
The solution is called Exothermic solution .
NaOH ( s ) + H2O ( l ) → Na+ ( aq ) + OH− ( aq )
Δ H° sol = – 51 kJ / mol
Standard heat of solution
The quantity of heat absorbed or released on dissolving one mole of the solute in a certain amount of the solvent to obtain a saturated solution under standard conditions .
The heat of solution can be calculated by the following relation :
qp = m . c . Δ T
Where , In diluted solutions , the solution mass ( m ) can be expressed by the volume , because the density of water in normal conditions equals 1 g / cm3 .
The specific heat of the diluted solution can be expressed by the specific heat of water 4.18 J/ g . ° C .
If the volume of the produced solution is ( 1 L ) and the amount of solute is ( 1 mol ) , the heat change in this case is called Molar heat of solution .
Molar heat of solution
The heat change resulting from dissolving one mole from the solute to form one litre of the solution , If the amount of solute is greater than 1 mole , we can calculate the molar heat of solution as the following :
Molar heat of solution ( Δ H sol ) = the amount of absorbed or released heat during dissolving ( qp ) ÷ number of moles of solute ( n )
Δ H sol = qp / n
Explanation of the source of the heat of solution
Dissolving process is affected by three forces which are : Attraction force between molecules ( particles ) of solvent , Attraction force between molecules ( particles ) of solute and Attraction force between particles of solvent and solute , So , the dissolving process takes place in three steps which are :
Separating solvent’s molecules from each other
Endothermic process , where an amount of energy ( Δ H1 ) is absorbed to overcome the attraction force between the molecules of the solvent with each other .
Separating solute’s particles from each other
Endothermic process , where an amount of energy ( Δ H2 ) is absorbed to overcome the attraction force between the particles of the solute with each other .
Dissolving process
Exothermic process , where , an amount of energy ( Δ H3 ) is released when solvent molecules are combined with the particles of solute .
The value of heat of solution Δ H° sol equals to the sum of these three energies :
Δ H° sol = Δ H1 + Δ H2 + Δ H2
If the solvent is water : Dissolving process is called Hydration , The energy released from dissolving process is called Hydration energy .
Hydration energy is the attaching of the dissociated ions with water molecules .
The hydration energy of silver ions equals – 510 kJ/mol means that the amount of energy released from attaching 1 mol of silver ions with water molecules equals – 510 kJ .
The type of dissolving process ( exothermic or endothermic ) is determined by the value of Δ H° sol .
If ( Δ H1 + Δ H2 ) < Δ H3 , So , Δ H° sol < 0 ( Negative sign ) and the dissolving is Exothermic .
If ( Δ H1 + Δ H2 ) > Δ H3 , So , Δ H° sol > 0 ( positive sign ) and the dissolving is Endothermic .
The standard heat of solution of lithium bromine equals – 49 kJ/mol means that the heat released from dissolving 1 mol of lithium bromine in an amount of solvent to get a saturated solution of it ( in standard conditions ) equals – 49 kJ .
The molar heat of solution of silver iodide equals + 84.4 kJ/mol means that the heat absorbed from dissolving 1 mol of silver iodide in an amount of solvent to form 1 L of a solution equals 84.4 kJ .
Standard heat of dilution Δ H° dil
When 1 mol of sodium hydroxide NaOH ( s ) dissolved in two different amounts of water H2O ( l ) , the heat of solution differs as the amount of water differs , as the following equations :
NaOH ( s ) + 5H2O ( l ) → NaOH ( aq ) , Δ H1 = – 37.8 kJ/mol
NaOH ( s ) + 200H2O ( l ) → NaOH ( aq ) , Δ H1 = – 42.3 kJ/mol
It is observed that the amount of released energy increases by adding another amount of water , The released or absorbed energy that resulted from adding another amount of solvent ( dilution ) is called Standard heat of dilution Δ H° dil .
Standard heat of dilution Δ H° dil is the quantity of released or absorbed heat for each one mole of solute when diluting the solution from a high concentration to another lower concentration with the condition of being in its standard state .
The standard heat of dilution of sodium hydroxide solution is – 4.5 kJ/mol means that the heat released from each 1 mol of sodium hydroxide solution , when it is diluted from a higher concentration to lower concentration at standard conditions equals 4.5 kJ , Dilution process takes place in two opposite steps , according to the energy :
Separation energy ( Endothermic process )
Because during dilution , the number of molecules of water increases and separate the ions or molecules of the solute from each in the concentrated solution , which needs an amount of energy .
Attaching energy ( Exothermic process )
Because the ions or molecules of the solute are attached to a greater number of molecules of the solvent , which leads to releasing an amount of energy , The heat of dilution is the sum of those two energies ( separation and attaching ) .
Heat Changes accompanying Chemical changes and Hess’s law of constant heat summation