Heat Changes accompanying Chemical changes and Hess’s law of constant heat summation
There are many forms of the heat changes accompanying the chemical reactions like Standard heat of combustion and Standard heat of formation , Combustion is the fast combination between the substance and oxygen .
Standard Heat of combustion ΔH°c
The complete combustion of some elements and compounds produces a large amount of energy in the form of heat or light or both of them ,The released energy is known as Heat of combustion ΔHc .
Heat of combustion ΔHc is the quantity of heat liberated ( released ) on the complete combustion of one mole of substance in an excess amount of oxygen , If the combustion takes place under standard conditions , It is called Standard heat of combustion ΔH°c .
Standard heat of combustion ΔH°c is the quantity of heat released when one mole of substance is completely combusted in an excess amount of oxygen under the standard conditions .
The combustion of organic substances ( like fuel and glucose ) produces water ( H2O ) , Carbon dioxide ( CO2 ) and Heat energy .
The standard heat of combustion of glucose equals – 2808 kJ/mol , This means that the heat released when one mole of glucose is completely combusted in an excess amount of oxygen under standard conditions equals 2808 kJ .
Combustion of stove gas ( Butagas )
Stove gas ( Butagas ) is a mixture of propane C3H8 and butane C4H10 , This combustion reaction produces a large amount of heat which is used in cooking food and it has other uses .
C3H8 ( g ) + 5 O2 ( g ) → 3CO2 + 4H2O ( g ) + 2323.7 kJ/mol
C3H8 ( g ) + 5 O2 ( g ) → 3CO2 + 4H2O ( g )
Δ H°c = – 2323.7 kJ/mol
Combustion of glucose inside the body of living organism
It is one of the very important combustion reactions as it provides the living organisms with the needed energy to perform their vital processes .
C6H12O6 ( s ) + 6 O2 ( g ) → 6CO2 + 6H2O ( g ) , Δ H°c = – 2808 kJ/mol
Standard heat of formation Δ H°f
The heat change accompanying the formation of the compound from its constituent elements is called Heat of formation Δ H°f .
If the constituent elements are in their standard state , which is the most stable state of the matter at the standard conditions , therefore the heat change accompanies the formation of the compound is called Standard heat of formation Δ H°f .
Heat of formation Δ Hf is the quantity of released or absorbed heat during the formation of one mole of a compound from its constituent elements .
Standard heat of formation Δ H°f is the quantity of released or absorbed heat when one mole of a compound is formed from its constituent elements where these elements are in their standard conditions .
The heat of formation of any element is supposed to be zero in the standard conditions , If the standard heat of formation of glucose equals – 1260 kJ/mol , This means that the quantity of released heat when one mole of glucose is formed from its constituent elements at standard conditions equals 1260 kJ .
Standard heat of formation of glucose
6C ( s ) + 6 H2 ( g ) + 3 O2( g ) → C6H12O6 ( s ) , Δ H°f = – 1260 kJ/mol .
The heat of formation of a compound equals its heat content , The change in the heat content can be calculated using the values of standard heat of formation from the following relation :
Δ H = The sum of the heat of formation of the products – The sum of the heat of formation of the reactants
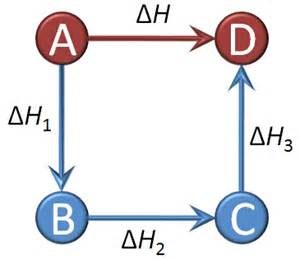
If the reaction is A + B → C + D
So , Δ H = [ heat of formation of C + heat of formation of D ] – [ heat of formation of A + heat of formation of B ]
Relation between heat of formation and the stability of the compound
The thermally stable compounds are Exothermic compounds , Their heat of formation has a negative sign because their heat content is lower than the heat content of their constituent elements , These compounds are thermally stable at room temperature and do not tend to dissociate to their elements .
The thermally unstable compounds are Endothermic compounds , Their heat of formation has a positive sign because their heat content is higher than the heat content of their constituent elements , These compounds are thermally unstable at room temperature and tend to dissociate spontaneously to their elements .
Most reactions move in the direction of the formation of the most stable compounds ( the lowest heat of formation ) .
The formation of 1 mol of HBr causes releasing of 36 kJ of heat , This means that HBr is a thermally stable compound ( Δ H°f = – 36 kJ / mol ) .
The formation of 1 mol of HI needs absorbing of 26 kJ of heat , This means that HI is a thermally unstable compound ( Δ H°f = + 26 kJ / mol ) .
The heat of formation of a compound is related to its stability because when the heat of formation of the compound decreases , its thermal stability increases and vice versa , So , most reactions move in the direction of the formation of the most stable compounds ( the lowest heat of formation ) .
Hess’s Law
Scientists usually prefer to use indirect methods to calculate heat of reactions , This is due to many reasons such as :
- Mixing of reactants or products with other substances .
- Some reactions occur very slowly and need a long time like the formation of rust .
- The presence of danger on measuring the heat of reaction experimentally .
- Difficulties of measuring the heat of reaction in normal conditions of pressure and temperature .
In order to measure the heat change of these cases of reactions , Hess proposed a law which is known as Hess’s law of constant heat summation .
Hess’s law of constant heat summation
Heat of reaction is a constant amount in standard conditions , whether the reaction is carried out in one step or a number of steps .
Hess’s law is dealing with the chemical equations as if they were algebraic equations that can be added together , subtracted from each other or multiplying its coefficient in a constant coefficient .
So , Hess’s law is very important due to its ability to calculate the change in the heat content of the reactions which can not be measured in a direct way .
The mathematical relation of Hess’s law is: Δ H = Δ H1 + Δ H2 + Δ H3
Atoms components, Rutherford and Bohr’s Atomic Models
Balanced chemical equations, Law of conservation of matter (mass) & Law of constant ratios