General properties of Benzene, How the detergents remove spots and dirties
Aromatic Benzene is used as an organic solvent for fats in dry cleaning, It is used in the manufacture of dyes, explosives and drugs, It is used in the manufacture of insecticides as gamixane, D.D.T. was used as insecticide & it was prevented in many developed countries, TNT is used as an explosive compound, The aromatic sulphonic acid compounds are used mainly in the detergent industry.
Nomenclature of benzene derivatives
The name of mono-substituted benzene is derived from the name of the atom or the group that is attached to the benzene ring followed by the word benzene, The entering atom or group attacks any carbon atom of benzene since the six carbon atoms in benzene ring are identical.
If benzene is trisubstituted, it is impossible to use the expression Ortho, Meta and Para, The carbon atoms in the ring take the numbers where each carbon atom attached to a group or atom takes the same number, the nomenclature is arranged according to alphabetical letter, for example, the bromine takes a number before chlorine before nitro group and so on and the sum of numbers must be the lowest.
Nomenclature by the IUPAC system, Direction of numbering to obtain the lower numbers, Arrange the branches alphabetically, Consider only the numbering of benzene, Aryl radical (Ar) is the radical which produce by removing one hydrogen atom from the aromatic compound (its symbol is Ar), for example when we remove a hydrogen atom from benzene, the produced radical is called phenyl radical (C6H5−).
Directing group
The entering atom or the group attacks any carbon atom of benzene since the six carbon atoms in the benzene ring are identical, The disubstituted benzene is represented by three isomers, They are Ortho (O−), Meta (m−) and Para (p−), The product depends on the nature of the substituted group which is mainly found (A), There are some groups direct the new substituent to Ortho and Para positions and other direct to Meta positions.
Direct the incoming substituent in the Ortho and Para positions, If the Directing group is the alkyl group (R), hydroxyl group (OH), amino groups (NH2) and halogen atoms (X) such as (Cl, F, Br, I), Direct the incoming substituent in the meta directing groups, If the Directing group is aldehydic group, (−CHO), ketonic group (−CO), Carboxylic group (−COOH), and Nitro group (−NO2).
Halogenation of nitrobenzene gives only one product while halogenation of toluene gives two products because the nitro group in nitrobenzene is meta-directing only while the methyl group in toluene is ortho, para directing group, The reaction of nitrobenzene with chlorine doesn’t form ortho-chloro nitrobenzene because nitro group directs to the meta position.
Physical properties of benzene
- It is a transparent liquid with a pleasant smell, boils at 80°C and it burns in air with a black smokey flame, this means its molecule contains a large number of carbon atoms.
- It is immiscible (insoluble) with water, miscible (soluble) with organic solvents.
- It dissolves some organic compounds, so, it is used in dry cleaning.
Chemical properties of benzene
Benzene reacts by two kinds of chemical reactions (addition and substitution).
Addition Reactions
Although benzene contains double bonds, its addition reaction is difficult and takes place under certain conditions, Benzene contains 3 double bonds, so, we add 3 molecules.
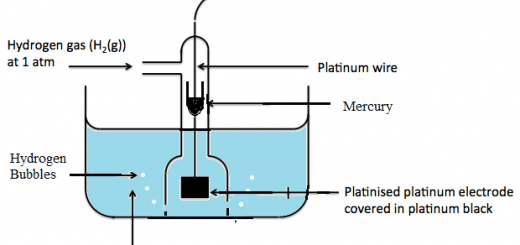
Addition of hydrogen (Hydrogenation): Benzene reacts with hydrogen under pressure and temperature, in the presence of a catalyst to give cyclohexane.
C6H6 + 3H2 → C6H12 (cyclohexane)
Halogenation: Benzene reacts with chlorine or bromine in direct sunlight to form hexahalocyclohexane, When it reacts with chlorine, an insecticide is formed which is called hexachlorocyclohexane (gamixane).
C6H6 + 3Cl2 → C6H6Cl6 (gamixane)
Halogenation is the reaction of benzene with chlorine gas in direct sunlight and in the presence of a catalyst, Benzene reacts by addition and substitution as it has three pi bond, so it reacts by addition and reacts with substitution because it has six replaceable hydrogens, The products of benzene halogenations depend on reaction conditions because in direct sunlight, the reaction type is addition, while in indirect sunlight the reaction type is substitution.
Substitution Reactions
Substitution reactions are very important reactions as they produce very important economical compounds, In these reactions, one or more hydrogen atoms are replaced by another atom or group.
Hydrogenation in indirect sunlight: One or more hydrogen atoms in the benzene ring can be replaced by the halogen in the presence of a catalyst, So one product is formed starting from monochloro to six benzene chloride.
C6H6 + Cl2 → C6H5Cl + HCl
In the previous reaction, One hydrogen atom is replaced by one chlorine atom, when chlorine reacts with benzene in indirect sunlight in the presence of iron III chloride as a catalyst to form chlorobenzene.
Aryl halides are produced in a large scale and are used as insecticides, the most important one is D.D.T. which is dichloro, diphenyl, trichloroethane (C14H9Cl5), Its poisonous effect is due to the presence of the group (CH−CCl3) which dissolves in the fatty tissue of the insect and kill it.
D.D.T. was manufactured in 1939 before the second World War, It was used in a great extent because of its severe poison on all kinds of insects which affect the plant and the animal and even the insects that affected soldiers during the war.
D.D.T. was called the ugliest compound in the history of chemistry for its environmental problems which appeared during its usage, It remains in the environment without decaying, killing useful insects such as bees.
Besides, it goes with rains to the rivers and lakes and kills fish and other aquatic creatures, it drains to food chain until it reaches the man, that is why it was prevented in many developed countries, but it is still used in other countries.
Alkylation (Friedel-Craft’s reaction): Benzene reacts with alkyl halides (RX) where the hydrogen atom of benzene ring is replaced by an alkyl group forming alkyl benzene, this reaction takes place in the presence of anhydrous aluminium chloride (AlCl3) as a catalyst.
C6H6 + CH3Cl → C6H5CH3 + HCl
Friedel-Craft’s reaction (Formation of toluene) is the reaction of benzene with methyl chloride in the presence of a catalyst to produce toluene (methyl benzene), The catalyst may be (AlCl3, FeCl3, ZnCl2), and toluene can be prepared by catalytic reformation of heptane.
Benzene prefers substitution reactions than addition reactions, Because in addition reactions, Benzene ring will be destroyed, so its aromatic property will be lost, while in substitution remains as it is.
Nitration: The reaction of benzene with conc. nitric acid in the presence of conc. sulpheric acid, where the hydrogen atom of benzene ring is replaced by a nitro group (−NO2), nitro benzene is formed, The role H2SO4 is to absorb water and prevent the reversible reaction.
C6H6 + HNO3→ C6H5NO2 + H2O
Polynitro organic compounds are very explosive substances because their molecules contain their own fuel, which is carbon besides oxygen which is the oxidizing agent, these compounds burn rapidly, and a great amount of heat and gases are produced accompanied by explosion, and this due to the weakness of the bond between N and O (N−O) to form the two strong bonds between C and O (C−O) in carbon dioxide and the bond between nitrogen atoms (N−N) in nitrogen molecule.
T.N.T (Trinitrotoluene) is one of the explosive nitro-organic compounds that was produced by millions of tons through the second world war, and still produced until now, this compound is prepared by the reaction between toluene and the mixture of concentrated nitric & sulphuric acids (1:1 ratio).
C7H8 + 3HNO3→ C7H5O6N3 + 3H2O
Sulphonation is the process in which the hydrogen atom of benzene ring is replaced by sulphonic acid group (SO3H), this usually takes place by the reaction between aromatic benzene and conc. sulphuric acid where benzene sulphonic acid is formed.
C6H6 + H2SO4→ C6H5SO3H + H2O
Importance Aryl sulphonic acid components
The detergent industries depend on the aromatic sulphonic acid compounds after the treatment with the caustic soda to obtain the water-soluble sodium salt, The molecule of detergent is composed of two parts: Head (polar) is an ionic group which is hydrophilic and Tail (Non-polar) is a long hydrophobic carbon chain.
The head of the detergent molecule is hydrophilic as it is an ionized group, so, it is attracted to the polar water molecules, Aromatic sulphonic acid compounds are important organic compounds that are used mainly in the manufacture of detergents.
How the detergents remove the spots and dirties
Pure water does not remove dirties because grease which is saturated with dirties is insoluble in water because they are organic where water is polar compound, so soapless detergents are used, this may explain the role of soapless detergent in removing the spots and dirties as follows:
- Adding detergent to water decreases the surface tension of water and it increases the ability of water to wet textile.
- When the detergent is dissolved in water, their molecules arrange themselves where the hydrophobic tail of each molecule is directed towards the dirties and adhere with them while the hydrophilic head is directed towards the water, so, the detergent molecules surround the dirties completely, the dirties completely covered by molecule.
- The friction produced by mechanical rubbing causes the separation of dirties and breaks them into small balls.
- The separation of small balls is the result of repulsion force between the heads of molecules (similar charges) and suspend in water in the form of emulsion which is removed by the rinsing process.
Cycloalkanes, Aromatic hydrocarbons, Preparation of benzene in industry and lab
Hydrocarbon derivatives, Ethanol, Alcohols Nomenclature, Classification and uses