First transition elements properties, electronic configuration and oxidation states
The transition element is the element in which the orbitals of d or F are occupied with electrons but not completely filled either in its atomic state or in one of its oxidation states, Transition elements have elements that have several oxidation numbers but representative elements mainly have one oxidation state.
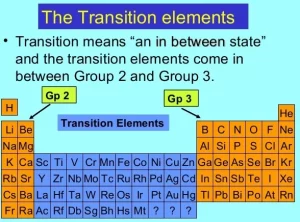
First transition elements
The first transition series is a group of 10 elements in the periodic table that are found in the 4th period. These elements are characterized by having their outer electron configuration in the 3rd orbitals. They are also known as the 3rd transition metals. The elements in the first transition series:
- Scandium (Sc).
- Titanium (Ti).
- Vanadium (V).
- Chromium (Cr).
- Manganese (Mn).
- Iron (Fe).
- Cobalt (Co).
- Nickel (Ni).
- Copper (Cu).
- Zinc (Zn).
Properties of the first transition series
- Variable oxidation states: These elements can exhibit multiple oxidation states due to the availability of unpaired electrons in their d orbitals.
- Colored compounds: Many of their compounds are colored due to d-d transitions.
- Catalytic activity: They are often used as catalysts in various chemical reactions.
- Magnetic properties: Some of them exhibit magnetic properties, such as ferromagnetism.
- Metallic character: They are all metals with typical metallic properties.
Applications of the first transition series
- Iron: Used in steel production, construction, and other applications.
- Copper: Used in electrical wiring, plumbing, and other applications.
- Zinc: Used in galvanizing, batteries, and other applications.
- Nickel: Used in batteries, alloys, and other applications.
- Titanium: Used in aerospace, medical implants, and other applications.
The first transition series is an important group of elements with a wide range of applications and properties.
Electronic configuration and oxidation states
The oxidation numbers increase in the first transition series from Sc to a maximum in Mn then decrease again to Zn with an exception for the elements of group I–B because from Sc to Mn, the number of unpaired electrons increases and from Mn to Zn pairing of electrons takes place.
The elements of the first transition series are located in the fourth period after calcium 20Ca whose electronic configuration is [18Ar] 4S2, after that there is a gradual filling of the five orbitals of (3d) sublevel by a single electron in each orbital in sequence till manganese (3d5), After manganese pairing of electrons takes place in each orbital till zinc (3d10) (Hund’s rule).
Chromium and copper are abnormal from the expected electronic configuration as their configurations are 24Cr (Ar) 3d5 4S1, and copper 29Cu (Ar) 3d10 4S1, in these elements, we find that their outermost sublevel 4s and 3d in chromium are half-filled while in copper 4s is half filled but 3d is filled, this explains that the atom has energy, this means that it has extra stability when the outermost sublevel is half filled (d5) or filled (d10) but this is not the only main factor that causes the stability of the atom.
Chromium 24Cr and copper 29Cu have abnormal electronic configurations because in 24Cr one electron will transfer from 4S to 3d, so, the sublevels become half filled and this makes the atom more stable and in 29Cu one electron will transfer from 4S to 3d, so, the sublevel 4S becomes half filled and 3d sublevel becomes filled and this makes the atom more stable.
The maximum oxidation number of any element can’t exceed its group number, the only deviation is noticed for group I–B elements (Cu, Ag, Au), On oxidation (when the atom loses electrons) the atom loses the first electrons of 4S (having high quantum number), followed by the electrons of 3d (lower quantum number) to give the different oxidation states.
Transition elements are characterized by having many oxidation states, due to the sublevel 3d is not completely filled and the sublevels (3d and 4S) are nearly equal in energy, so, when the atom gains a gradual amount of energy, the two electrons of the sublevel 4S lost firstly, then the electrons of the sublevel 3d in sequence, as the difference in ionization energies are small.
The atom or the ion becomes stable when the sublevel d is:
- Empty (d0) as in the case of Sc+3 and Ti+4.
- Half filled (d5) as in the case of Mn+2 and Fe+3.
- Completely filled (d10) as in the case of Zn+2.
Iron (II) is easily oxidized to iron (III), while Manganese (II) isn’t easily oxidized to Manganese (III), iron (III) ion is more stable as the 3d sublevel is half-filled 3d5, so the reaction goes towards the formation of the more stable compound, but in case of manganese atom, the electronic configuration is 25Mn (Ar) 3d5, 4S2, The 3d sublevel is half-filled 3d5 in Mn+2 ion, so, it is more stable than Mn (III) ion, So Mn+2 ion is not readily oxidized to Mn+3 ion.
Fe+2 [18Ar], 3d6 (oxidation) → Fe+3 [18Ar], 3d5
Mn+2, 3d5 → Mn+3, 3d4
It is difficult to oxidize iron (III) ion into IV ion, while it is easy to oxidize Titanium (III) ion into Titanium IV because the sublevel (3d) is half-filled in Fe+3 and empty in Ti+4 which makes the element more stable.
26Fe : [18Ar] 3d6, 4S2 Fe+3 [18Ar], 3d5 (oxidation) → Fe+4 [18Ar], 3d4
22Ti : [18Ar] 3d2, 4S2 Ti+3 [18Ar] , 3d1 (oxidation) → Ti+4 [18Ar], 3d0
All elements of the first transition series have an oxidation state (+2) because after losing the electrons of (4s) sublevel at first (except for scadium), while in the higher oxidation states they lose the electron of (3d) in sequence.
The oxidation states increase from scandium to manganese which has the highest oxidation state (+7) in group VIIB, after that the oxidation states decrease gradually to be (+2) in zinc in group IIB, So, the maximum oxidation state does not exceed its group number except for the group IB that contains copper, silver and gold.
The main transition elements are characterized by several oxidation states, this can be explained as following when the atom of transition elements oxidized by losing electrons from 4s and 3d sublevels in sequence (which are close in energy), therefore the ionization potentials for the transition element increases gradually.
Representative metals like sodium, magnesium and aluminum have one oxidation state due to the increase in the second ionization potential of sodium and the third of magnesium and the fourth of aluminum is very high because we need high energy to break down a complete energy level, so, it is difficult to obtain Na+2 or Mg+3 or Al+4 during the chemical reaction under normal condition.
Scandium doesn’t produce scandium ion Sc+4 by normal chemical methods, scandium has only one oxidation state (Sc+3) because the difference between the third I.P and fourth I.P is very large due to breaking of a complete energy level.
Scandium gives (+3) oxidation state not (+2), because the difference between the 2nd and 3rd ionization potential is small, so scandium loses two electrons from 4S sublevel than one electron from 3d sublevel to be more stable, i.e. the stability of Argon.
Scandium doesn’t form compounds in which scandium has an oxidation state (+2) because when giving scandium energy, it loses (3) electrons, this is because the energy of sublevel (4S) is near from energy of sublevel (3d), so, atom becomes more stable and electronic configuration of scandium reach to the nearest inert gas.
Coinage metals copper 29Cu (3d10 4S1), silver 47Ag (4d10 5S1), and gold 79Au (5d10 6S1) are considered transition elements because, in case of (+2) or (+3), the (d) sublevel contains 9 or 8 electrons, so it is occupied by electrons, but not completely filled.
Metals such as zinc 30Zn (4S2, 3d10), Cadmium 48Cd (5S2, 4d10), and Mercury 80Hg (6S2, 5d10) are not considered as transition elements because the (d) sublevel is completely filled (d10) in the three metals in both atomic state and oxidation state (+2) ion, so they are not transition elements.
Potassium has only one oxidation state while vanadium has more than one oxidation state because in K, the increase in the I.P is very large due to breaking a complete energy level, but vanadium has more than one oxidation state because the two sublevels 4S and 3d have nearly equal energies, so, they lost their electrons in sequence when the atom is oxidized.
19K : [18Ar] , 4S1
23V : [18Ar] , 4S2, 3d3
The elements of first transition series are characterized by a variety of its oxidation states as the two sublevels (4S) and (3d) have nearly equal energy and their electrons are lost in sequence when the atom is oxidized.
Transition elements & Economic importance of elements of the first transition series
General properties of the first transition elements in the modern periodic table