Extraction of Iron from its ores, Iron dressing, Reduction & production
Iron is the most important metal in heavy industries, Iron is the fourth most abundant element in Earth’s crust after oxygen, silicon and aluminum as it forms (5.1 %) of the mass of the Earth’s crust and this mass increases gradually as we come close to the center of earth, Iron occurs only in the form of pure metal (90%) in meteorites.
Iron
Iron ( 26Fe: (18Ar), 4S2, 3d6 ) is found in the earth’s crust in the form of natural ores which contains different iron oxides mixed with impurities such as Silica (SiO2), (Al2O3), CaO, and MgO, and some harmful impurities such as s, p and As, The suitability of the ore in the extraction of iron economically depends on three factors which are:
- The iron percentage in the ore.
- The composition of impurities in the ore.
- The type of harmful elements mixed with the ore as sulphur (S), phosphorus (P), and Arsenic (As) and others.
The ore is Hematite, chemical name is iron III oxide, the chemical formula is Fe3O4, It has a blood red colour, it is more easily reduced, Iron is from 50-60 %, place of deposits is Oasis area (western desert) and western part of Aswan.
The ore is Limonite, chemical name is Hydrated iron III oxide, the chemical formula is 2Fe2O3.3H2O, It has a yellow hydrated oxide & it is easily reduced, Iron is from 20-60 %, place of deposits is Oasis area.
The ore is Magnetite, chemical name is the magnetic iron oxide, the chemical formula is Fe3O4, It is a black ore having magnetic properties, Iron is from 45-70 %, place of deposits is Eastern desert.
The ore is Siderite, chemical name is Iron II carbonate, the chemical formula is FeCO3, It is has a yellowish grey colour & it is easily reduced, Iron is from 30-42 %.
Magnetite is a compound behaves as a mixture of two oxides: FeO (iron II oxide, the oxidation number of iron = +2), Fe2O3 (iron III oxide, the oxidation number of iron = +3), so, the oxidation number of Fe in Fe3O4 is (+2, +3), Magnetic iron oxide is a mixed oxide because when it reacts with conc. acids, two types of salts are produced.
Fe3O4 + 8 HCl → FeCl2 + 2FeCl3 + 4 H2O
Extraction of Iron from its ores
Extraction of iron or its metallurgy is the process of obtaining this metal in a form where it can be put to practical use, and this process of extraction consists of three stages: Ore dressing, Reduction of ores and Iron production.
The ore dressing
Ore dressing aims to increase the concentration of iron in the ore by removing the unwanted impurities and improving the properties of the ore which helps in the successive stages of extraction, The ore dressing process is carried out to improve the physical and mechanical properties of iron ore and includes Crushing process, Sintering process, Purification and concentration of the ore.
The crushing process is used to obtain iron ore in small sizes that can be reduced easily, Sintering process is used to obtain the fine particles of iron ores in large sizes, As a result of the crushing process & cleaning furnace a huge amount of fine particles of ore are obtained which can not be used directly in high furnace, these particles must be treated to collect them in a larger size to be similar & homogeneous and this process is called sintering.
The sintering process is the process of treatment of the fine particles of iron ore obtained from the crushing process or in a cleaning furnace to collect them in a larger size to be similar and homogeneous particles fit for the reduction process.
The purification and concentration process is the process of using surface tension properly, and magnetic or electrical separation to remove the unwanted impurities which are chemically combined or mixed with ore to increase the percentage of iron in the ore.
The ore-dressing process is also carried out to improve the chemical properties of ores by roasting, It means heating the substance strongly in the air to dry the ore, expelling humidity, converting the iron ore into oxide, increasing the ratio of iron in the ore, oxidation of some harmful impurities as (S and P).
2 FeCO3 → 2 FeO + 2 CO2
2 FeO + ½ O2 → Fe2O3
2 Fe2O3.3H2O → 2 Fe2O3 + 3H2O
Oxidation of some impurities such as sulphur and phosphorus
S + O2 → SO2
4 P + 5 O2 → 2 P2O5
Roasting is the process of heating iron ore strongly in dry air to dry the ore and expelling humidity, It is used to increase the percentage of iron in the ore and for the oxidation of some impurities such as sulphur and phosphorus.
Roasting of iron is very important in the ore dressing process but this process pollutes the environment, Ore dressing is important for iron ores before their reduction to remove most impurities and improve the physical & chemical properties of the ore.
It is very important to dress iron ores before reduction as the iron ore dressing improves the physical, mechanical and chemical properties of the ores and makes it suitable to be reduced easily and effectively, The disappearance of the luster of a piece of iron when it is heated because the iron tends to form a layer of iron oxide on its surface after heating.
Reduction of iron ores
The reduction process is the process of reducing iron oxides to iron by carbon monoxide resulting from coke in the blast furnace or by a mixture of carbon monoxide and hydrogen gases (water gas) resulting from natural gas in the Midrex furnace.
In this stage, the reduction of iron oxides to iron is carried out by one of two methods depending on the reducing agent which may be:
- CO gas resulting from coke as a Blast furnace.
- The mixture of (CO + H2) resulting from natural gas as in Midrex furnace.
Reduction by using carbon monoxide resulting from coke in the blast furnace
C + O2 → CO2
CO2 + C → 2 CO
3 CO + Fe2O3 → 2 Fe + 3 CO2
Midrex Furnace to obtain Spongy Iron
Reduction of Fe2O3 by using a mixture of carbon monoxide and hydrogen (water gas) that is produced from natural gas (93% methane) in the Midrex furnace to produce Spongy iron, Spongy iron is the iron mixed with impurities where it is produced from the midrex furnace and it has holes similar to that in the sponge.
2CH4 + CO2+ H2O → 3 CO + 5 H2
3 CO + 2 Fe2O3 + 3 H2→ 4 Fe + 3CO2 + 3H2O
Iron production
After the reduction of iron ores in the blast furnace or Midrex furnace, the third step in which the production of different types of iron such as cast iron and steel, The steel industry depends on two essential processes:
- Removal of unwanted impurities from iron resulting from the reduction furnaces.
- Addition of some elements to iron to produce steel with the required properties for industrial purposes.
The production of steel may be formed by one of the following furnaces: oxygen converters, Open-hearth furnace, and Electric Furnace.
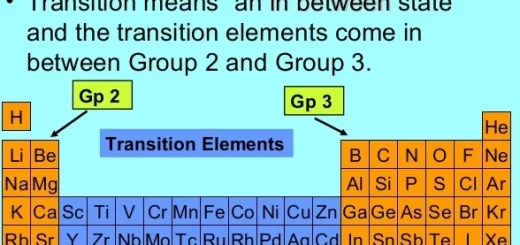
General properties of the first transition elements in the modern periodic table
Iron alloys, oxides, preparation, types, properties and importance