Elements classification, Mendeleev’s, Moseley’s and Modern periodic table
Elements of the group (1A) are known as alkali metals because they react with water forming alkaline solutions. Potassium is more active than sodium because the atomic size of potassium is larger than that of sodium. Elements of the same group have similar properties because their atoms have the same number of electrons in the outermost energy level.
Elements
What are the most important attempts to classify elements?
Many attempts are made by scientists for the classification of elements according to their properties to facilitate their study and find a relationship between elements and their chemical and physical properties. The most important attempts are Mendeleev’s periodic table, Moseley’s periodic table, and the Modern periodic table.
Mendeleev’s periodic table
It is the first real periodic table for classifying elements. At this time, only 67 elements were known. Mendeleev explained his periodic table in his book “Principles of Chemistry” in 1871.
How did Mendeleev classify elements?
He prepared 67 cards, each card represented an element. He recorded on each card: The symbol of each element, its atomic weight, and its important properties (boiling point, melting point, density, the formula of oxide, …). He arranged elements of similar properties in vertical columns which were known later as groups. He classified the elements of each main group into two subgroups (A), (B) due to the differences between their properties.
He discovered that: The elements are arranged in ascending order according to their atomic weights on moving from the left side of the table to the right side in horizontal rows which were known later as periods, The properties of elements were repeated periodically by the beginning of each new period.
Advantages of Mendeleev’s table
- He predicted the discovery of new elements and determined the values of their atomic weights, therefore he left gaps (empty cells) in his table.
- He corrected the atomic weights of some elements, which were estimated wrongly.
Mendeleev left gaps (empty cells) in his periodic table because he predicted the discovery of new elements and determined the value of their atomic weights. In 1871, Mendeleev predicted the properties of an unknown element and named it ICA silicon. This element was discovered later in 1886 and was named Germanium (Ge) and its properties were the same as Mendeleev had predicted.
Disadvantages of Mendeleev’s table
- He had to make a disturbance in the ascending order of atomic weights of some elements to put them in groups that suit their properties.
- He had to deal with isotopes of one element which were later discovered as different elements due to the difference in their atomic weights.
Mendeleev had to put more than one element in one cell as iron, cobalt, and nickel due to the similarity in their properties. Isotopes are many forms of atoms of one element having the same atomic number but differ in atomic weight such as the isotopes of hydrogen: 11H, 21H, 31H.
Moseley’s periodic table
(In 1913), The Newzealand scientist Rutherford discovered that the nucleus of the atom contains positively charged protons, In the same year, the English scientist Moseley named the term of “atomic number” of an element on the number of positive protons in the nucleus of its atom.
He discovered after studying X-rays properties that the periodicity of the properties of the elements is related to their atomic numbers and not their atomic weights as Mendeleev believed. Among the discoveries which have helped Moseley to put his periodic table are:
- Radiation activity phenomena.
- Getting of X-rays.
- More knowledge about the arrangement of electrons in atoms.
The most important modifications of Moseley on Mendeleev’s table
- He arranged elements in ascending order according to their atomic numbers. where the atomic number of each element increases by one from the preceding element in the same period.
- He added to the table the zero group which includes inert (noble) gases, and the other elements that were discovered after the preparation of Mendeleev for his periodic table.
- He specified a place below the table for lanthanides and actinides elements.
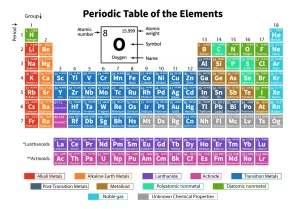
Modern periodic table
The recent studies led to the identification of the accurate composition of the atom, where: The Danish scientist Bohr had discovered the main energy levels of the atom and their number reaches 7 in the heaviest known atoms till now, Scientists had discovered that each main energy level consists of a definite number of other levels known as energy sublevels.
Accordingly, The elements were reclassified in a new table known as “Modern periodic table” in which the elements are classified in ascending order according to their atomic numbers and the way of filling the energy sublevels with electrons. Each main energy level consists of some sublevels equal to its number.
The number of known elements in the modern periodic table till now is 118 elements, 92 elements are abundant (available) in Earth’s crust, while the rest are prepared artificially under special conditions. The recently discovered elements do not exist in nature, but they are prepared artificially from other elements, They are radioactive elements, whose nuclei disintegrate in less than a second.
Elements are classified in ascending order according to:
- Their atomic weights in Mendeleev’s periodic table.
- Their atomic numbers in Moseley’s periodic table.
- Their atomic numbers, and the way of filling the energy sublevels with electrons in the Modern periodic table.
Description of the Modern Periodic Table
The modern periodic table consists of 7 horizontal periods each period stars by filling a new energy level, and 18 vertical groups, where each group has a traditional number and a modern number. The modern periodic table is classified into 4 main blocks which are: s-block, p-block, d-block, and f-block.
s-block
It is located on the left side of the periodic table, It consists of two groups, All groups of s-block take the letter “A”, It includes the two groups (1A) and (2A).
p-block
It is located on the right side of the periodic table, It consists of six groups, All groups of p-block take the letter “A” except zero group (18) “Noble gases” It starts with groups 3A (13) and ends with zero groups (18).
d-block
It is located in the middle of the periodic table, It consists of ten groups. All groups in the d-block take the letter “B” except group (8) which contains 3 vertical columns (groups), It starts to appear from the period (4), It starts with group 3B (3), and ends with group 2B (12), The elements of d-block are known as transition elements, It separates between s-block elements (left side of the table) and p-block elements (right side of the table).
f-block
It is located below the periodic table, It consists of two horizontal series which are lanthanides and actinides.
The atomic number = number of electrons = number of protons
The number of electrons that saturate the first four energy levels (K, L, M, and N) can be calculated from the relation (2n2) where (n) is the number of energy level, therefore:
- K level is saturated with 2 electrons.
- L level is saturated with 8 electrons.
- M level is saturated with 18 electrons.
- N level is saturated with 32 electrons.
How to determine the location of elements of groups (A) in the periodic table by knowing their atomic numbers?
- The number of energy levels occupied by electrons {Indicates} —> The periodic number of the element
- The number of electrons in the outermost energy levels {indicates} —> The group number of the elements (according to the traditional number)
To determine the location of 20Ca in the modern periodic table, the Number of energy levels occupied with electrons = 4 energy levels, So, the element is located in period (4), the number of electrons in the outermost energy level = 2 electrons, So, the element is located in the group (2A).
The outermost energy level of all noble gases is completely filled with 8 electrons except helium with 2 electrons because it contains only one energy level (K level). Helium (2He) is located in zero group, and isn’t located in group (2A) because it has only one energy level (K) which is saturated with 2 electrons, so, it is a noble gas that is located in zero group (group 18).
Elements of the same group’s
They are similar in their chemical properties because they are similar in the number of electrons in the outermost energy level, They are different in the number of energy levels occupied by electrons.
Magnesium (12Mg) and calcium (20Ca) are similar in their properties because they are located in the same group (2A), so, they are similar in the number of electrons in the outermost energy level (2 electrons).
Elements of the same period
They are different in their chemical properties because they are different in the number of electrons in the outermost energy level, They are similar in the number of energy levels occupied by electrons.
Aluminium (13Al) and chlorine (17Cl) are located in the same period in the periodic table because they are similar in the number of energy levels occupied by electrons (3 energy levels).
How to determine the atomic number of elements of groups (A) by knowing their location in the periodic table:
Determine the number of energy levels occupied by electrons by knowing the period number of the element, The number of electrons in the outermost energy level by knowing the group number of the element (according to the traditional number).
Write the electronic configuration of the atom of the element taking into consideration that the inner energy levels are completed with electrons. Then calculate the sum of the numbers of electrons that revolve in energy levels, and it represents the atomic number of the element.
i.e. The number of electrons that revolve in energy levels = The number of protons inside the nucleus of an atom = The atomic number of the element.
To calculate the atomic number, Element (X) is located in the second period and in group (7A), It is located in period (2), so, its electrons are distributed in two energy levels. It is located in group (7A), so, the outermost energy level contains 7 electrons, then the atomic number of element (X) = 2 + 7 = 9.
To calculate the atomic number, Element (Y) is located in the third period and in zero group, It is located in period (3), so, its electrons are distributed in three energy levels. It is located in zero group, so, the outermost energy level contains 8 electrons, then the atomic number of the element (Y) = 2 + 8 + 8 = 18.
In the modern periodic table
In the same group, the atomic number of an element increases from the element preceding it by (8). [except lithium element (3Li) that its atomic number increases that hydrogen (1H) by (2)]. In the same period, The atomic number of an element increases from the element preceding it by (1).
Scientists cannot discover a new element between sulphur (16S) and chlorine (17C) because the atomic number of an element is an integer and it increases in the same period from one element to the following element by one.
Classification of elements in Long-form periodic table, Ionization Energy & Oxidation numbers
Metallic & nonmetallic property, Acidic & basic property in the periodic table
Transition elements & Economic importance of elements of the first transition series
Radius property, Ionization potential, Electron affinity & Electronegativity