Atoms components, Rutherford and Bohr’s Atomic Models
Matter is composed of atoms , These atoms show the physical and chemical properties of the matter , By the end of the nineteenth century , Scientists had become sure that electrons are from the main components of atoms , Electrons are negatively charged particles of a very small mass .
Since the atom is electrically neutral , So , the atom has other particles carrying positive charges equal to the negative charges of the electrons , However , There wasn’t a known distribution for the positive and the negative charges in the atom at that time .
Rutherford and Bohr’s Atomic Models
According to the experiment of Rutherford in 1911 and Bohr’s theory in 1913 , the atomic structure became more acceptable .
Rutherford’s atomic models
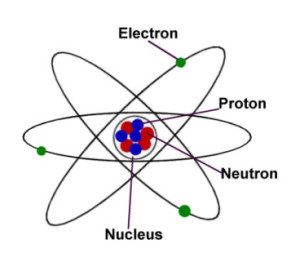
A heavy nucleus carries a positive charge in the centre of atom , Negative electrons rotate around the nucleus at a relatively far distance , Most of the atomic volume is space , The nucleus’s diameter = 10−6 : 10−5 nm , while the atom’s diameter = 1 × 10−10 m ( 0.1 nm ) , The mass of the atom is concentrated in the nucleus .
Bohr’s atomic models
The negatively charged electrons rotate around the nucleus in certain fixed orbits called energy levels , Each energy level is occupied by a certain number of electrons that can’t be increased .
Discovery of proton
Rutherford proved in 1919 that the nucleus of atom contains positively charged particles called protons , where the mass of proton is larger than that of the electron by 1800 times approximately .
Discovery of neutrons
Nevil Sidgwick discovered in 1932 that the nucleus contains particles that neutrally charged called neutrons , where the mass of neutron is nearly similar to the mass of proton .
The atom’s mass is concentrated in the nucleus because the mass of electrons is too small ( negligible ) compared to the mass of protons and neutrons ( mass of protons = 1800 times of electron mass ) .
The atom is electrically neutral because the number of positive charged protons in the nucleus equals to the number of negative charged electrons rotating around the nucleus .
Mass number and Atomic number
To describe the nucleus of the atom of any element , you should know Mass number ( A ) , Atomic number ( Z ) and Neutrons number ( N ) , Mass number ( A ) is the sum of the numbers of protons and nucleus of the element atom .
Atomic number ( Z ) is the number of protons inside the nucleus of the element atom , Number of protons = Number of electrons , Neutrons number is N , N = A – Z , Nucleons are the protons and neutrons inside the nucleus .
Isotopes
Isotopes are the atoms of the same element that have the same atomic number ( Z ) , but differ in mass number ( A ) due to the difference in the number of neutrons inside their nuclei .
The isotopes have the same chemical properties because they have similar number of electrons and the same electron configuration around the nucleus , Most elements in the periodic table have more than one isotope .
Hydrogen isotopes
Hydrogen is the simplest element in the nature , It has three isotopes , Name of isotopes are Protium , Deuterium and Tritium , The atomic number equals the mass number in protium nucleus because it does not contain neutrons , Number of neutrons equals the number of protons in deuterium nucleus , while it equals double the number of protons in tritium nucleus .
Oxygen isotopes :
Oxygen element has three isotopes which are Oxygen-18 , Oxygen-16 , Oxygen-17 , In nuclear chemistry , other nuclear terminology is used in addition to the isotopes , which are :
- Isobars are the nuclei of atoms of different elements that have the same number of mass ( A ) , but they differ in the atomic number ( Z ) .
- Isotones are the nuclei of atoms of different elements that have the same number of neutrons , but they differ in the mass number .
Atomic mass units amu ( u )
Masses of atomic isotopes are too small , So , They are measured in atomic mass units amu or u = 1.66 × 10−24 g = 1.66 × 10−27 kg , Atomic masses of elements can be identified by knowing the relatively atomic masses of their isotopes and the ratio of the presence of each of them .
Relationship between mass and energy
Calculation of the produced energy from the transforming of a mass of a substance ( estimated in kilograms kg ) to energy ( estimated in Joule J ) .
E = m . c²
E = Energy ( J ) , m = transformed mass ( kg ) , c = light speed ( 3 × 108 m/s )² .
Calculation of the produced energy from the transforming of a mass of a substance ( estimated in atomic mass unit u ) to energy ( estimated in million electron volt unit MeV ) .
E = m × 931
E = Energy ( Me V ) , m = Mass ( u ) , 931 = constant value .
Properties of Strong nuclear forces , Source of nuclear binding energy and Quark Model
Evolution concept of the atomic structure , Atomic theory & Properties of cathode rays
Atomic emission spectra , Bohr’s atomic theory , Wave mechanical theory of the atom