Ostwald’s Law, Hydronium Ion and Applications of the law of mass action on ionic equilibrium
Ionic equilibrium is the equilibrium between the molecules of a weak electrolyte and its ions, On Ionic equilibrium applied only on week electrolyte not strong electrolyte because in week electrolyte incomplete ionization takes place (there is an equilibrium between the molecules and ions of week electrolyte), while, in case of strong electrolyte complete ionization takes place.
Applications of the law of mass action on ionic equilibrium
- Electrolytic solution
- Ionization of water and pH value
- Hydrolysis of salt solution
- Solubility product
Electrolytic solution
According to ionization in water, Substances are classified into:
- Non-electrolytic substances: These are substances that do not ionize when dissolved in water, so, they do not conduct electricity as covalent compounds (sugar as glucose C6H12O6, starch).
- Electrolytic substances: These are substances that ionize when dissolved in water, so, they conduct electricity and can be classified into two types which are strong electrolyte and weak electrolyte.
Strong electrolyte: “Complete ionization”, All unionized molecules are changed into ions such as strong acids, strong alkali, and salts.
HCl → H+ + Cl−
NaCl → Na+ + Cl−
NaOH → Na+ + OH−
Weak electrolyte: “Incomplete ionization”, Do not ionize completely in water such as weak acids and weak alkali.
CH3COOH → CH3COO−+ H+
In weak electrolytes, only a small ratio of molecules is ionized because there are two opposite processes occurs at the same time, dissociation of molecules into ions and the combination between ions to form molecules, when the rates of the two processes are equal, a state of equilibrium is reached.
The aqueous solution of hydrochloric acid is a good conductor of electricity, but that of acetic acid is a weak one because of hydrochloric acid from the strong acids which are completely ionized while acetic acid from weak acids which are incompletely ionized.
Ionization is a process in which unionized molecules are changed into ions, Complete ionization is a process in which all unionized molecules are changed into ions which happens in strong electrolytes, Incomplete ionization is a process in which a small fraction of molecules is ionized which happens in weak electrolytes.
Some substances in the solid state contain ions such as sodium chloride, when ionic substances are dissolved in water (+ve) and (− ve) are liberated from their crystal structure and their solutions conduct the electricity, these substances are known as ionic compounds whose (+ve) and (− ve) ions are attracted to each other by electrostatic attraction forces.
On the other hand, in the case of covalent compounds, such as hydrogen chloride gas and pure ethanoic acid, the bonds between their atoms in a molecule are covalent, these two substances are also ionized in water but with different degrees, Hydrogen chloride gas is ionized, nearly 100% and ethanoic acid undergoes ionization at much less extent, this can be verified by the following experiments.
Experiment (1)
Test the electrical conductivity of pure ethanoic acid, and of hydrogen chloride gas dissolved in benzene, you can notice that the lamp in both cases will not illuminate indicating that neither of the two liquids contains ions to conduct the electric current.
Experiment (2)
Dissolve 0.1 mole of hydrogen chloride gas in one liter of water, also dissolve 0.1 mole of pure ethanoic acid in water to make two acid solutions of equal concentration, Test the electrical conductivity of each solution, You can notice that in the case of hydrochloric acid, the lamp gives a strong illumination, but gives a faint illumination with ethanoic acid, indicating that the first solution contains little ions.
Experiment (3)
Test for the effect of dilution on both solutions mentioned above, and such effect on the conductivity of electric current ( as indicated by the strength of illumination of the lamp), Dilute each solution to 0.01 molar and test the electrical conductivity, then further dilute each solution to 0.001 molar and test the conductivity of each solution again, You will notice that the illumination of the lamp is not affected in the case of dilution of hydrochloric acid, but the illumination increases in the case of the dilution of acetic acid.
Conclusion
Covalent compounds such as dry hydrogen chloride and pure ethanoic acid ionize in water, Ionization of hydrogen chloride is complete, but that of acetic acid is very limited so, the acidic solution of HCl is a good conductor of electricity whereas the ethanoic acid solution is a bad conductor, Also the extent of ionization of hydrochloric acid is not affected by dilution, but the extent of ionization of ethanoic acid increases by dilution, indicating the presence of more ionized molecules of the acid.
Hydronium Ion
Hydronium ion is the positive ion produced from the combination of the water molecule with the positive hydrogen ions, No free hydrogen ion (proton) is present in the aqueous solution of ionized acids, this ion is attracted to the lone pair of electrons on the oxygen atom of the water molecule and connected to a water molecule by a coordinate bond, this proton is called hydronium ions or hydrated proton or hydroxonium ion [H3O]+.
HCl + H2O → [H3O]+ + Cl−
H2O + H+→ H3O+
Two opposite cases are always found in the solution dissociation of molecules into ions, and the combination between the ions to give molecules, A state of equilibrium is achieved between ions and molecules as represented by the following equation.
AB → A+ + B−
Weak electrolyte → Dissociated ions
This type of equilibrium is known as ionic equilibrium, Ionic equilibrium is a state of equilibrium arising between molecules of a weak electrolyte and ions resulting from it, The law of mass action can be applied only in the case of weak electrolyte solutions, Strong electrolytes do not contain un-dissociated molecules, since they are completely ionized.
Ostwald’s Law
Ostwald described the relation between the degree of ionization of weak electrolyte (alpha α) and concentration for the solution (C) mole/litre, A monoprotic weak acid (HA) dissociates in water according to the equation.
HA → H+ + A−
By applying the law of mass action on this equilibrium system, the following relation is obtained.
Ka = [H+] [A−] / [HA]
Where [H+], [A−] represent the concentration of the ions produced, [HA] represents the undissociated acid at the equilibrium state, Ka is the ionization or dissociation constant of the acid, When one mole of a weak acid (HA) dissolves in (V) liter of the solution, then at equilibrium.
Degree of ionization = Number of dissociated moles/ Total number of moles before
If the number of dissociated moles is (α) mole then, the number of undissociated moles from HA = (1−α) and the number of moles of (H+) and (A−) produced equals α mole, The concentration of the substance (mole/liter.
The concentration (C) = Number of moles / Volume by liter (V)
Ka = α²/ [ V (1−α) ]
In weak (monobasic or monoprotic) acid:
- The degree of ionization (α) is directly proportional to dilution.
- The degree of ionization (α) is inversely proportional to concentration.
- The degree of ionization (α) = Number of dissociated moles of acid (after H2O)/ Total number of moles before dissociation (before H2O)
If the number of moles before dissociation is 1 mole, therefore, the degree of ionization (α) is equal to the number of dissociated moles of acids, Ostwald’s law for dilution illustrate the quantitative relation between the degree of ionization (α) and dilution.
Ostwald’s law: At a constant temperature, the product of multiplication ions produced divided by the concentration of unionized moles for weak electrolytes, is constant and is called ionization constant.
At a constant temperature, the degree of ionization (α) increases by dilution (so that Ka value remains constant) In the case of weak electrolytes, the degree of ionization (α) is small enough and can be neglected, consequently the value (1−α) is considered approximately one and the relation becomes, Ka = α²/ V.
Since the concentration of the weak acid (C) = 1/V mole /liter, the above equation becomes Ka = α² × C
This means that increasing dilution (decreasing concentration) causes an increase in dissociation degree and vice versa.
Ostwald’s law: Ionization constant (Ka) for a weak acid with a concentration (C) can be determined from the relation, Ka = α² × C, where α is the degree of ionization.
Calculation of hydronium ion concentration of weak acids
When a weak acid such as acetic acid with a concentration (C) dissociates in water according to the equation
CH3COOH + H2O → CH3COO− + H3O+
The dissociation constant for such reaction, Ka = 1.8 × 10−5
From the above equation, the quantity of acetate ion (CH3COO−) released equals the quantity of hydronium ion (H3O+) produced.
Since the acid is weak, the amount dissociated (α) is small and can be neglected, so, the concentration of acetic acid at equilibrium (C−α) equals the original acetic acid concentration (C) consequently.
[ H3O+]= (C× Ka)½
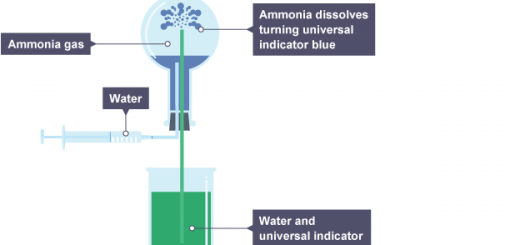
Weak bases are the bases that are partially dissociated in aqueous solutions when ammonia, a weak base, is dissolved in water, the following equilibrium reaction takes place:
NH3 + H2O → NH4+ + OH−
This equation reveals that one of both NH4+ and OH− ions is formed, Since the dissociation constant of ammonia is small, a small portion of ammonia dissociates and at equilibrium, the concentration of the remaining ammonia [NH3] equals the original concentration of ammonia (Cb).
[OH−] = (Cb × Ka)½
The ionization constant of acids (Ka) indicates their strength, As the degree of ionization of acid is directly proportional to the ionization degree according to this relation α² = Ka / Ca and keep Ka constant or because the strength of the acid is directly proportional to the ionization constant Ka.
Ionization of water, Hydrogen Exponent (pH value), Solubility product & Hydrolysis of salt solutions
Chemical bonds, Ionic bonds, Properties & types of covalent bonds