Alkanes (Paraffins), Methane and Nomenclature of organic compounds
Alkanes are open-chain aliphatic hydrocarbons in which the carbon atoms are combined together with a single bond of sigma type, They are saturated, Methane is the first member, It is considered as the simplest organic compound, The world paraffin is a Latin word that means unreactive.
Alkanes (Paraffins)
They are saturated open chain Aliphatic Hydrocarbons (Paraffins), They have mono covalent bonds between C atoms, Their reactions by replacement, All alkanes are characterized by the general formula (CnH2n+2), where (n) is the number of carbon atoms, Total number of atoms = 3n+2.
Alkanes are relatively chemically inactive because the carbon atoms are combined together by a single bond called the sigma bond which is strong and difficult to break, Each compound exceeds the previous one by (– CH2) methylene group, Fractional distillation is a method that is used to separate alkanes from each other in petroleum ore.
Alkanes are ended by the suffix (ane) which means that the compound is belonging to (alkanes chain), the prefix of the name indicates the number of carbon atoms in the molecule, for example, the prefix Meth = 1, Eth = 2, Prop = 3, But = 4, Pent = 5 and so on, Alkanes are from a homologous series.
Homologous series are compounds that have the same general molecular formula, chemical properties, and graduated physical properties and each compound exceeds the previous one by (– CH2) group, such as Alkanes, Alkenes, and Alkynes.
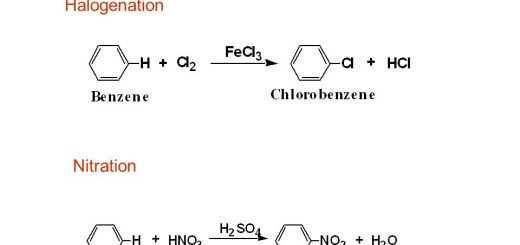
Radical is an organic atomic group that can’t be found alone but it must be connected with another element or group, The aryl radical (Ar) is a radical that is produced by removing one hydrogen atom from the aromatic compounds, When a hydrogen atom is removed from benzene, the radical produced is called phenyl radical (C6H5–).
The Alkyl Radical (R–) is an organic group which does not found alone, It is derived from the corresponding alkane by removing one hydrogen atom, Alkyl radicals are given the symbol “R”, Their general formula is (CnH2n+1), Its name is derived from the corresponding alkane by replacing the suffix (ane) by (yl), such as Methyl (CH3), Ethyl (C2H5 ), Propyl (C3H7) and Butyl (C4H9).
Nomenclature of organic compounds
IUPAC system means the International Union of Pure and Applied Chemistry, It is a method used for the nomenclature of organic compounds depending on the number of carbon atoms in the longest continuous carbon chain.
What is the difference between the common name and the IUPAC name of organic compounds?
- The common name: the nomenclature of the organic compounds depends on their sources in nature.
- The IUPAC: The nomenclature of the organic compounds follows the IUPAC which enables everyone to identify the precise of construction of this compound.
Nomenclature of Alkanes (IUPAC system)
Anciently alkanes were called paraffins (Common name), The name of the hydrocarbon is determined according to the longest continuous carbon chain which may be linear or branched, If the longest hydrocarbon chain is free from any branches or side chain, the carbon atoms are given numbers from any side (left or right side).
If the longest hydrocarbon chain is attached to an alkyl group or any other atoms except hydrogen, the numbering of carbon atoms in the hydrocarbon chain begins from the side which is nearer to the branch, the nomenclature begins by the number of the carbon atom from which the chain arises, then the name of the branch, and ending by the name of the alkane.
If the side is repeated in the hydrocarbon chain, we use prefix Di– or Tri– or Tetra– to indicate the number of repetition, If the branch is a halogen atom such as chlorine or bromine or a group such as Nitric NO2− , So its name is ended by the letter O, so we say chloro, bromo or nitro, If the side groups are different (alkyl group and halogens), the groups are arranged according to their alphabetical Latin names and the sum of numbers must be the lowest value.
Unsaturated compounds (double or triple bond)
Start numbering from the nearest side to the double bond or triple bond, If an alkyl radical is attached to an unsaturated hydrocarbon.
Methane (CH4)
Methane is considered the simplest organic compound forms about 50 – 90% of natural gas found under the earth’s crust or accompanied by crude petroleum, It is found in coal mines that may be exposed to an explosion as a result of its illumination.
Methane is sometimes called the gas of swamps because it goes out as bubbles from the bottom of these swamps as a result of decaying of the organic matters, It is found in atmospheric air (about 0,00022%).
It is believed that it was the main component of the atmospheric air of Earth, The atmospheric air was made of methane (CH4), ammonia (NH3), H2 and water vapour (reducing agents) but by the time, they were exposed to UV, They reached and changed into O2 and N2 which change the atmosphere from reducing atmosphere to oxidizing atmosphere which helps in the burning process.
Preparation of methane gas in the lab
Methane can be prepared in the lab, by dry distillation of anhydrous sodium acetate with soda lime (NaOH, CaO ), Soda Lime is a mixture of NaOH (caustic soda) and quicklime (CaO) which doesn’t take part in the reaction but it helps in reducing the melting points of the reaction mixture.
CH3COONa(s) + NaOH(s) → CH4 (g) + Na2CO3(s)
Dry distillation is a process that is used in the preparation of methane in the lab, The apparatus is free from the air because the mixture of methane and air burn with the explosion, Soda Lime is preferred than caustic soda to prepare methane by dry distillation of sodium acetate because CaO is used to decrease the melting point of sodium acetate and to absorb water vapour.
Properties of Methane
- It doesn’t remove the colour of bromine solution as it does not react by addition due to the absence of double bond (π).
- It is tasteless, colourless and odourless.
Organic chemistry, Properties of Organic and inorganic compounds
Alkanes economic importance, use, physical & chemical properties