Classification of lipids, Simple lipids & fatty acids, Triacylglycerols & importance of true fats
Lipids are heterogeneous groups of organic compounds related, either actually or potentially to fatty acids, they contain C, H, and O, they may also contain P, N, and S. Lipids play many important roles in maintaining the health of an organism, the most important function of lipids is as building blocks of cellular membranes, Other functions include energy storage, insulation, cellular communication, and protection.
Lipids
Lipids are a group of macromolecules, they can store energy, signal between cells, and form the cell membrane. They are made from monomers called fatty acids, They are not soluble in water, making them important in cell function. Unlike water, Lipids are non-polar, which means they won’t mix with water.
Lipids are referred to as hydrophobic, which means “water-fearing.” If you mix oil & water, they remain separate because oil is a lipid & non-polar. They have the common property of being:
- Relatively insoluble in water.
- Soluble in nonpolar solvents (fat solvents) as ether, chloroform, benzene, and acetone. They can be extracted from the cells by these non-polar solvents.
- They can be utilized by living organisms.
- They are molecules that contain fatty acids or derived from fatty acids compounds.
Classification of lipids
1- Simple lipids:
- True fats: esters of fatty acids with glycerol.
- Waxes: esters of fatty acids with higher molecular weight monohydric alcohol.
2- Complex lipids (compound lipids): They are esters of fatty acids with alcohol and in addition, they contain another group (e.g. phosphate, carbohydrate, sulphate, and protein).
3- Derived lipids:
- Fatty acids.
- Alcohols, as glycerol, sphingosine, cholesterol, and higher alcohols of waxes.
- Steroids.
- Carotenoids.
- Fat-soluble vitamins: Vitamins A, D, E, and K.
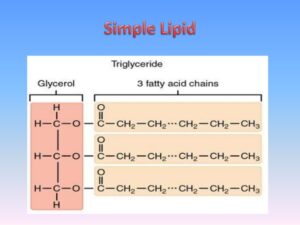
Simple lipids (True fats)
True fats (Neutral fats): These are esters of glycerol and various fatty acids, if all the three hydroxyl groups of glycerol are esterified, fats are known as triacylglycerol, the 3 fatty acids linked to the glycerol are usually different. Glycerol is trihydric alcohol containing three hydroxyl groups, it is colorless, syrupy liquid, sweet, and very hygroscopic.
Fatty acids are aliphatic monocarboxylic organic acids, they are obtained mostly from the hydrolysis of natural fats and oils. Fatty acids that occur in neutral fats usually contain an even number of carbon atoms, ranging from 2-24 carbon atoms, all of them possess a long hydrocarbon chain and a terminal carboxyl group.
Classification of fatty acids
A. According to the chain length of fatty acids they can be:
1- Low fatty acids (short-chain fatty acids): i.e. Containing 10 carbon atoms or less (from 2-10), such as:
- Acetic acid (2C): CH3-COOH
- Butyric (4C): CH3-CH2-CH2-COOH
2- High fatty acids (long-chain fatty acids): Contain more than 10 carbon atoms, such as:
- Palmitic (16 C): CH3-(CH2)14-COOH
- Stearic (18 C): CH3-(CH2)16-COOH
B. Fatty acids can be classified according to saturation and unsaturation:
1- Saturated fatty acids such as:
- Butyric fatty acids
- palmitic
- stearic
2- Unsaturated fatty acids: contain one or more double bonds, they can be classified into:
a. Monosaturated fatty acids contain one double bond, e.g.:
- Oleic acid 18: 1; 9 (ω 9)
- Nervonic acid 24: 1; 15 (ω 9)
b. Polyunsaturated fatty acids: contain more than one double bond, usually the double bonds are separated by methylene groups (-CH2-), e.g.:
- Linoleic 18: 2; 9, 12 (ω 6)
- α-Linolenic 18: 3; 9 12, 15 (ω 3)
- Arachidonic 20: 4; 5 8, 11, 14 (ω 6), it is a precursor to prostaglandins.
Various ways are used for indicating the number and position of the double bonds, e.g.: in oleic acid 18: 1; 9 or Δ9 18:1 or ω9, c 18:1, Δ9 this means a double bond between carbon 9 and 10 counting from the carboxylic group. CH3 (CH2)7 CH = CH (CH2)7-COOH, ω9 means a double bond on carbon atom no. 9 counting from the methyl group. CH (CH2)7 COOH.
C. Biological classification of fatty acids:
From the nutritional point of view fatty acids can be classified into:
- Essential fatty acids: These are polyunsaturated fatty acids. i.e. contain more than one double bond, they are: Linoleic(ω6), and Linolenic(ω3). Linoleic and linolenic can not be synthesized by mammals and must be obtained from plant sources.
- Non-essential fatty acids: Those which are saturated fatty acids or FA contain one double bond, mammals can synthesize them from other precursors.
- Relatively essential e.g. Arachidonic acid (ω6), arachidonic found in animal fats and peanut oil, and is synthesized in the body from linoleic acid. Arachidonic acid becomes essential if its precursor, linoleic acid is missing in the diet.
Triacylglycerols may be:
- Simple triacylglycerols: i.e. contain a single kind of fatty acids in all the three ester positions. e.g. Tristearin, Tripalmitin, and Triolein.
- Mixed triacylglycerols: i.e. contain 2 or more different fatty acids in the molecule, e.g. 1,3- Distearopalmitin
Biological importance of true fats
- They form reserve foods in animals and plants, In animals, they are found as depot fat in the subcutaneous tissues, this depot fat is mobilized during starvation to produce energy and so its amount is variable, and thus true fats are known as the variable element of fat.
- They are the most compact form in which energy can be stored. (1 gm of fat → 9.3 KCal).
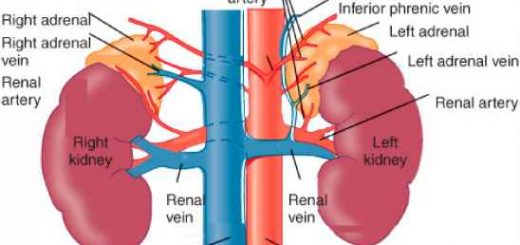
- True fats are also found as supporting material in some parts of the body as around the kidneys.
Complex Lipids function, definition, types & structure, Phospholipids & Glycolipids
Types of Derived lipids, Steroids, Animal sterols, Plant sterols & bile acids
Carbohydrates, Polysaccharides, Proteoglycans, Glycoproteins & Glycosaminoglycans
Carbohydrates importance, Types of Isomerism, Monosaccharides & Disaccharides
Connective tissue cells types, function & structure, Resident cells & Transient cells
Skin appendages types, function (hair, nails, sweat glands & sebaceous glands)

how many acids would you need to name per poly, mono, and unsaturated fatty acids?