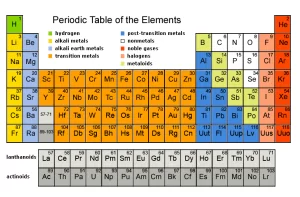
Main Groups in Modern Periodic Table, Alkali metals, Halogens and Inert gases groups
Alkali metals are monovalent elements because they tend to lose the valency electron during the chemical reaction. the elements of group (1A) are known as alkali metals because they react with the water forming alkaline solutions. Potassium is more active than sodium because the atomic size of potassium is larger than that of sodium.
Modern periodic table groups
Some of the main groups in the modern periodic table are characterized by specific names due to their properties such as Alkali metals, Halogens, and Inert gases. Group (1) or (1A) is called the “Alkali metals” group, Group (17), or (7A) is called the “Halogens” group, Group (18), or (Zero groups) is called the “Inert gases” group.
Alkali metals group [Group 1A]
Alkali metals group is located on the maximum left side of the modern periodic table. It is the first group of s-block, Despite the presence of hydrogen at the top of the group (1A), It is not one of the alkali metals but it is one of the nonmetals because it has a small atomic size and it is a gas.
General properties of alkali metals
Physical properties
- All of them are solids at ordinary temperature and they have a metallic luster.
- They are good conductors of heat and electricity.
- Most of them have low density.
The lowest density of alkali metal is lithium (Li) element. The highest-density alkali metal is the Cesium (Cs) element. Alkali elements that float on the water surface: Lithium (Li), Sodium (Na), and Potassium (K) because their densities are smaller than the density of water (1 gm/cm³).
Alkali elements that sink in water: Rubidium (Rb), Cesium (Cs) because their densities are greater than the density of water (1 gm/cm³). Lithium floats on the water surface, while cesium sinks in water because the density of lithium is less than that of water, while the density of cesium is greater than that of water.
Chemical properties
- Their outermost energy level contains only one electron.
- They are monovalent elements because they tend to lose their valency (outermost) electrons during the chemical reaction forming positive ions. each of them carries one positive charge.
- They are chemically active elements so, they are kept under the surface of kerosene or paraffin oil to prevent their reactions with moist air. Alkali metals are kept under the surface of kerosene [except lithium which is kept under the surface of paraffin oil].
- Their chemical activity increases as the atomic number increases due to the increase of their atomic sizes, so they can lose the valency electron easily, Cesium (Cs) is the most active alkali metal in the periodic table because it has the largest atomic size so, it can lose its valency electron easily.
Lithium is not kept under the surface of kerosene, because it floats on its surface and burns at once causing the burning of kerosene too so, it is kept in paraffin oil as lithium sinks in paraffin oil.
Sodium is kept under the surface of kerosene to prevent it from the reaction with moist air as it is an active metal. Lithium (Li) is the least active metal in the group (1A) because it has the least atomic size in the group (1A).
Wrap two small pieces of sodium (pea size) and potassium with a filter paper, and put each of them carefully in a beaker containing water. Both sodium and potassium react strongly with water forming an alkaline solution and hydrogen gas evolves which burns with a pop sound (by the effect of the heat produced from the reaction). The reaction of potassium with water is stronger than that of sodium because potassium is more active than sodium.
2Na + 2H2O → 2NaOH + H2 ↑
2K + 2H2O → 2KOH + H2 ↑
Elements of the group (1A) in the periodic table are called alkali metals (alkaline metals) because they react with water forming alkaline solutions. Sodium fires are not put off with water, because sodium reacts instantly with water, and hydrogen gas evolves which burns with a pop sound by the effect of the heat of the reaction.
Halogens group [Group 7A]
Group 7A is located on the right side of the modern periodic table, It is one of the groups of p-block.
General properties of halogens
Physical properties
- They are bad conductors of heat and electricity.
- Their physical state is graduated from gaseous states (Fluorine and Chlorine), liquid state (Bromine), and solid-state (Iodine).
Chemical properties
- Their outermost energy level contains 7 electrons.
- They are monovalent elements because they tend to gain one electron only during the chemical reaction forming negative ions each of them carries one negative charge. Nonmetal “halogen” (M) + Electron (e–) → Negative ion (M–).
- They are chemically active elements, therefore they do not exist individually in nature but they exist in chemical compounds [except Astatine (At) which is prepared artificially].
- They exist in the form of diatomic molecules (formed of two atoms). The formula of halogen molecules is Fluorine (F2), Chlorine (Cl2), Bromine (Br2), and Iodine (I2).
- Halogens react with metals forming salts so, they are called halogens, which means “Salts formations”.
- Each element from halogens replaces the elements below it in their salt solution.
2K + Br2 → 2KBr
2Na + Cl2 → 2NaCl
Cl2 + 2KBr → 2KCl +Br2
Cl2 + 2NaBr → 2NaCl + Br2
Br2 + 2Kl → 2KBr + I2
Although fluorine is the most active halogen, it doesn’t replace other halogens in their salt solutions because it reacts with water which the salt dissolved in. Chlorine is used in the manufacture of the corrector, which is a very volatile liquid. On using it, it becomes dry quickly, leaving a white substance on the words and lines required to be deleted.
Inert gases group [Group 18]
Group 18 is located on the maximum right side of the modern periodic table, It is the last group in the p-block.
General properties of inert gases
- They are present in a gaseous state.
- Their outermost energy level contains 8 electrons except for Helium which contains only 2 electrons [Helium has only (K) energy level].
- Their valency equals zero because their outermost energy levels are saturated with electrons.
- They are chemically inactive elements, where they don’t react with other elements under normal conditions.
- They exist in the form of monoatomic molecules (formed of one atom only).
Properties of elements and their uses
The uses of elements or their compounds in modern techniques depend on their properties and types, The following table shows the uses of some elements.
- Sodium (Na), Sodium in a liquid state [Metal]. It is used in a liquid state (as it is a good conductor of heat) in transferring heat from inside the nuclear reactor to outside to be used to obtain the vapour energy required to generate electricity.
- Cobalt (Co), Radioactive cobalt 60 [Transition element] 60 is the mass number of cobalt. It is used in food preservation because it radiates (emits) gamma rays, which prevent the reproduction of microbial cells without an effect on the human.
- Silicon (Si), Silicon [Metalloid], Silicon slides are used in the manufacture of electronic devices such as computers and transistors because it is a semiconductor which its ability to conduct electricity depends on temperature.
- Nitrogen (N), Liquefied nitrogen [Non-metal]. It is used in the preservation of the cornea of the eye due to the decrease in its boiling point (-196°C)
The Egyptian scientist Dr: Moustafa El-Sayed got the highest American medal in science for his efforts in Nanotechnology on 29th September 2008 and applied this technology in using gold in the treatments of cancer disease.
Importance of Alkali Metals, Halogens, and Inert Gases
- Uses of Alkali Metals: Sodium is used in street lighting, Lithium is used in batteries, and Potassium is vital for biological functions, such as nerve signaling.
- Halogens uses: Chlorine can be used in water purification and disinfectants, Fluorine compounds can be used in toothpaste and non-stick cookware (Teflon), and Iodine is necessary for thyroid function in humans.
- Inert Gases uses: Helium can be used in balloons and as a coolant in MRI machines, Neon can be used in advertising signs (neon lights), and Argon can be used in light bulbs and as a protective gas in welding.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Chemical activity series, Chemical properties of metals & nonmetals
Graduation of the properties of the elements in the modern periodic table
Modern periodic table and classification of Elements
Elements classification, Mendeleev’s, Moseley’s & Modern periodic table
Classification of elements in Long-form periodic table, Ionization Energy & Oxidation numbers




This was very Helpful too!! Thank you Heba Soffar!!
You are welcome