Metalloids and the difference between the positive ion and negative ion
The metalloids
The metalloids are semi-metallic elements that have the properties of both metals and nonmetals, It is difficult to know the metalloids from their outermost electrons due to the difference in the numbers of the electrons in their valencies shells.
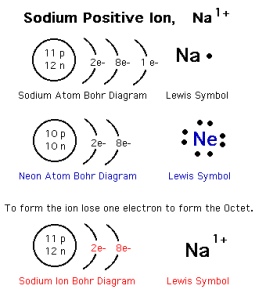
The positive ion
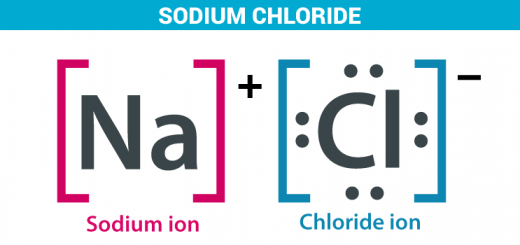
The positive ion (the cation) is the atom of a metallic element that loses one electron or more during the chemical reactions, The positive ion carries positive charges equal to the number of the lost electrons.
The number of the protons of the positive ion is more than that of its electrons, The electronic structure of the positive ion is similar to that of the nearest preceding inert gas.
The number of energy levels in the positive ion is less than the number of energy levels in its atom.
The negative ion
The negative ion (the anion) is an atom of a nonmetallic element that gains one electron or more during chemical reactions, The number of electrons of the negative ion is more than that of its protons.
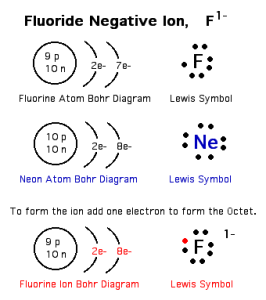
The number of energy levels in the negative ion is equal to the number of the energy levels in its atom.
The negative ion carries the negative charges equal to the number of the gained electrons.
You notice that the number of energy levels in the negative ion is equal to the number of energy levels in its atom.
The electronic structure of the negative ion is similar to that of the nearest inert gas that follows.
You can download Science online application on google play from this link: Science online Apps on Google Play
Metallic & nonmetallic property, Acidic & basic property in the periodic table
Radius property, Ionization potential, Electron affinity & Electronegativity