Chemical combination, Types of bonds (Chemical bonds and Physical bonds)
The most stable atoms are those of noble gases such as helium, neon, argon …..etc, Because their outermost energy level is filled with electrons, so, the atoms of these electrons do not undergo any chemical reaction ( at normal conditions ) with other elements or with each, their molecules are monoatomic.
Chemical reaction concept
All of the other elements are reactive to some extent, They undergo chemical reactions to complete their outermost shell by accepting, losing, or sharing some electrons to acquire an identical electron configuration as that of the nearest noble gas.
As a result of this change in the number of electrons in the outermost shells of atoms, bonds are formed between atoms or bonds are broken to form new bonds, this is called a chemical reaction, Chemical reaction is the breaking of bonds between atoms of the reactants molecules to form new bonds between atoms of the products molecules.
Application on the chemical reaction concept: The mixture of iron filings with sulphur powder is not considered a chemical compound, because there is no chemical reaction occurs between them, If this mixture is heated to a high temperature, a chemical reaction occurs (formation of chemical bond) between iron and sulphur producing the compound iron ( II ) sulphide .
Lewis electron-dot symbols
The valence electrons have an important role in the formed bond, So, the scientist Lewis had set a simple way to represent the valence electrons by using dots :
Application: Dot-representation of the valence electrons of the oxygen atom 8O
The electron configuration of oxygen atom: 1s², 2s², 2p4, At first, the valence electrons are distributed singularly on the four sides of the symbol, then pairing takes till completing the configuration.
The scientist Lewis had differentiated between the lone pair and bond pair of electrons, The Lone pair is the electron pair that is found in one of the outer orbitals and does not share in bond formation, Bond pair is the electron pair that is responsible for the bond formation.
Types of bonds
Bonds are divided into Chemical bonds (Ionic bond, Covalent bond, Coordinate bond) and Physical bonds ( Hydrogen bond, Metallic bond).
Chemical bonds
Ionic bond
This bond is usually formed between the elements of the two terminals of the periodic table which are :
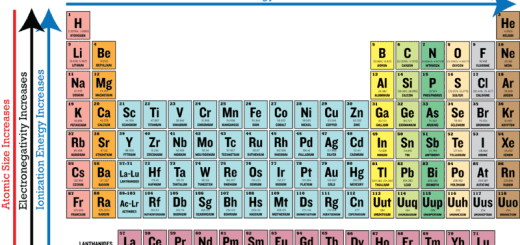
Metals: They are characterized by their large atomic volumes and their low ionization energies, Therefore, Their atoms tend to lose the electrons of the outermost shell and change into cations ( positive ion ) with an identical electron structure to the nearest noble gas precedes them in the periodic table.
Nonmetals: They are characterized by their small atomic volumes and their high electron affinities, Therefore, Their atoms tend to gain electrons ( those lost by metal atoms ) and change into anions ( negative ion ) with an identical electron structure to the nearest noble gas follows them in the periodic table.
Consequently, an electrostatic attraction occurs between the positive cations and the negative anions which is known as the ionic bond, The ionic bond has no materialistic existence.
The ionic bonding and electronegativity
The properties of compounds differ according to the difference in electronegativity between their elements, The difference in electronegativity between the bonded elements plays a role in the characteristics of the ionic bond, whereas the horizontal distance between the bonded elements – in the periodic table – increases, the difference in electronegativity increases, then the strength of ionic bond increases.
In general, when the difference in electronegativity is more than 1.7 then the formed compound is ionic, The ionic bond is the chemical bond that is formed between metals and nonmetals, the difference in electronegativity between them is more than 1.7 .
Molten sodium chloride conducts electricity more than molten magnesium chloride because the ionic bond in NaCl is stronger than that of MgCl2, where the difference in electronegativity between sodium and chlorine is greater than that between magnesium and chlorine atoms.
Although chlorine is a nonmetal and aluminum is a metal, aluminum chloride shows the properties of the covalent compounds, Because the difference in electronegativity between aluminum and chlorine atoms is less than 1.7.
Sodium chloride has high melting and boiling points, due to the strong ionic bond between sodium and chlorine atoms which is resulted from the high difference in electronegativity between them (2.1).
Covalent bond
The covalent bond is formed between atoms of nonmetals of the same electronegativity (atoms of the same element) or those of close electronegativities by sharing of electrons, where each atom shares by a certain number of electrons of the outermost shell equals the number of electrons required to complete this shell, forming a pair or more of electrons being in the vicinity of each atom.
Classification of covalent bond
The covalent bond is classified according to the difference in electronegativity between the bonded atoms into three types, which are: Pure covalent bond, Non-polar covalent bond, and Polar covalent bond.
Pure covalent bond
When the two bonded atoms are of the same nonmetal element (have the same electronegativity): Each atom in the molecule has the same ability to attract the two shared electrons (electron pair) of the bond, thus the electron pair spends the same time in the vicinity of each atom and the net charge for each of them equals zero, It is described as a pure covalent bond.
A pure covalent bond is a bond formed between two atoms, and the difference in electronegativity between them equals zero, Examples: The bond in the hydrogen molecule ( H − H ) and the bond in the fluorine molecule (F − F).
Non-polar covalent bond
When two atoms of different nonmetal elements are bonded, where the difference in electronegativity between them is greater than zero till 0.4, it is described as a non-polar covalent bond.
A non-polar covalent bond is the bond formed between two atoms of two different nonmetal elements, the difference in electronegativity between them is greater than zero till 0.4, Example: The bond in the methane molecule ( C − H ).
Polar covalent bond
When the difference in electronegativity between the bonded atoms of two nonmetal elements is somewhat high (greater than 0.4 and less than 1.7), the more electronegative atom has a greater attraction for the electron pair of the bond, so, the electrons spend more time in the vicinity of it.
As a result, the atom acquires a partial negative charge ( δ– ) – not a complete one – and because of this unequal sharing of the electron pair towards it, the less electronegative atom acquires a partial positive charge ( δ+ ), The produced molecule is then described as a polar molecule and the bond is described as a polar covalent bond.
A polar covalent bond is a bond formed between two atoms of nonmetal elements, the difference in electronegativity between them is greater than 0.4 and less than 1.7.
As the difference in the electronegativity between the bonded elements in the polar molecule increases, the strength of the covalent bond increases, For Example: The bond in the hydrogen chloride molecule, The bond in a water molecule, and The bond in the ammonia molecule.
Application: The polar covalent bond in the hydrogen chloride molecule HCl
In a hydrogen chloride molecule, the more electronegative chlorine atom has a greater attraction for the electron pair of the covalent bond, So, the electron pair spends more time in the vicinity of the chlorine atom.
As a result, the chlorine atom acquires the partial negative charge (δ–), As of this unequal sharing of the electron pair towards chlorine, the hydrogen atom acquires the partial positive charge (δ+).
The bond in water molecule is polar covalent, while in chlorine molecule is a pure covalent bond, because of the difference in the electronegativity between the hydrogen and oxygen atoms in water molecule is somewhat high (greater than 0.4 and less than 1.7), while the difference in the electronegativity between the two chlorine atoms in chlorine molecule equals zero.
NH3 molecule is polar, Because the difference in the electronegativity between nitrogen and hydrogen atoms (N − H) is greater than 0.4 and less than 1.7, also the sum of the polar pair of the molecule does not equal zero.
Hydroxy compounds, Rules for calculating the oxidation numbers
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept