Attempts of elements classification, Mendeleev, Moseley and Modern periodic table
The scientists classified the elements according to their properties to facilitate their study and to find a relationship between the elements, and their chemical and physical properties. The modern periodic table was developed by Dmitri Mendeleev and later refined with the discovery of atomic numbers, It is one of the most powerful tools in chemistry and science.
Mendeleev’s periodic table
There were only 67 elements that were known in Mendeleev’s periodic table, Mendeleev classified the elements in ascending order according to their atomic weights. Mendeleev left gaps in his table predicting the discovery of the new elements, and he corrected the atomic weights of some elements that were estimated wrongly.
But Mendeleev had to make a disturbance in the ascending order of the atomic weights of some elements to put them in the groups that suit their properties. Mendeleev had to deal with the isotopes of one element as different elements due to the difference in their atomic weights. So, He had to put more than one element in one cell of his table.
Moseley’s periodic table
Moseley classified the elements in ascending order according to their atomic numbers, He added the zero group which includes the inert gases to the table, and he specified a place below the table for lanthanides and actinides elements.
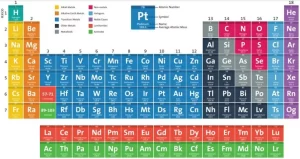
The modern periodic table
It is separated into rows, called periods, and columns, called groups or families, The elements in the same group have similar chemical and physical properties due to their similar electron configurations, It is an arrangement of chemical elements in a tabular form, It is organized based on their atomic number (number of protons), electron configuration, and recurring chemical properties.
The elements are classified in ascending order according to their atomic numbers, and the way of filling the energy sublevels with electrons, The number of known elements in the modern periodic table till now is 116 elements, and 92 elements are available in the Earth’s crust, while the rest are prepared artificially.
There are 7 horizontal periods, and 16 vertical groups (18 vertical columns), and the elements are classified into 4 blocks (s, p, d, and f). The elements of the same group are similar in the number of the electrons in outermost energy level. So, they are similar in their chemical properties, and they are different in the number of energy levels occupied by the electrons.
The elements of the same period are different in the number of the outermost electrons. So, they differ in their chemical properties. and they are similar in the number of energy levels occupied by the electrons.
Features of the Modern Periodic Table
Atomic Number: The elements are arranged in order of increasing atomic number (the number of protons in the nucleus of an atom), This replaced Mendeleev’s original arrangement by atomic mass.
Periods (Rows): The horizontal rows in the modern periodic table are called periods, There are 7 periods in the modern periodic table, As you move across a period from left to right, the atomic number increases, and elements change from metallic to non-metallic properties, The elements in the same period have the same number of atomic orbitals (energy levels). The elements in the second period have two energy levels.
Groups or Families (Columns): The vertical columns are called groups, The modern periodic table has 18 groups, The elements in the same group have the same number of valence electrons, which gives them similar chemical reactivity. all elements in Group 1 have 1 valence electron and are highly reactive metals, Groups are given special names:
- Group 1 is called Alkali metals (e.g., lithium, sodium).
- Group 2 is called Alkaline earth metals (e.g., magnesium, calcium).
- Group 17 is called Halogens (e.g., fluorine, chlorine).
- Group 18 is called Noble gases (e.g., helium, neon, argon).
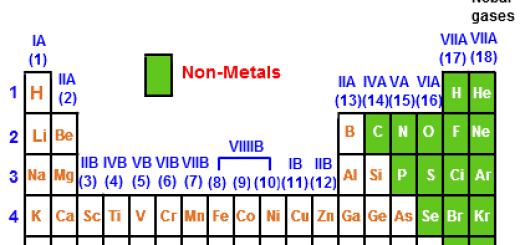
Metals, Nonmetals, and Metalloids
- Metals are located on the left side and in the center of the modern periodic table, Metals are shiny, conductive, and malleable (e.g., iron, copper).
- Nonmetals are located on the right side of the modern periodic table (except hydrogen, which is also a nonmetal). Nonmetals are poor conductors and brittle (e.g., oxygen, sulfur).
- Metalloids are located along the staircase-like line between metals and nonmetals. Metalloids have properties of both metals and nonmetals (e.g., silicon, and arsenic).
Blocks of the Periodic Table
The modern periodic table can be divided into blocks based on electron configurations:
- s-block: Groups 1 and 2 (and helium).
- p-block: Groups 13 to 18.
- d-block: Transition metals (Groups 3 to 12).
- f-block: Lanthanides and actinides (rare earth elements, located below the main body of the table).
Transition Metals
- The transition metals are located in the d-block, They have variable oxidation states and form colored compounds, These elements include familiar metals like iron, copper, and gold.
- Lanthanides and Actinides are elements that are part of the f-block, and they are placed separately at the bottom of the table to maintain the structure.
- Lanthanides (atomic numbers 57-71) are rare earth metals used in electronics and strong magnets.
- Actinides (atomic numbers 89-103) are mostly radioactive, with some like uranium and plutonium used in nuclear energy.
Periodic Trends
- Atomic Radius: Atomic size decreases across a period (left to right) due to increasing nuclear charge pulling electrons closer. Atomic size increases down a group because additional electron shells are added, increasing the distance between the nucleus and the outermost electrons.
- Ionization Energy is the energy required to remove an electron from an atom, It increases across a period due to stronger attraction between electrons and the nucleus. It decreases down a group because the outermost electrons are farther from the nucleus, making them easier to remove.
- Electronegativity is the ability of an atom to attract electrons in a chemical bond. It increases across a period and decreases down a group. Fluorine (Group 17, Period 2) is the most electronegative element.
- Metallic and Nonmetallic Character: Metallic character decreases across a period and increases down a group. Nonmetals, which are found on the right side of the table, tend to gain electrons in reactions, while metals (on the left) tend to lose electrons.
Importance of the Modern Periodic Table
The modern periodic table allows scientists to predict the chemical behavior of elements based on their position. This is useful in both academic research and practical applications, such as creating new materials or understanding chemical reactions, Elements are systematically classified, providing a clear understanding of how they relate to each other. The arrangement of the modern periodic table reflects periodicity in chemical properties, helping chemists to easily identify similarities and differences between elements.
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Graduation of the properties of the elements in the modern periodic table
Classification of elements in Long-form periodic table, Ionization Energy and Oxidation numbers
The graduation of the electronegativity of the elements in the periodic table