Boiling point and separation of the petroleum oil, Fractional distillation of crude oil steps
The boiling point is the temperature at which a matter begins to change from a liquid state to a gaseous state, The change of matter from the liquid state to the gaseous state is known as boiling, and the temperature at which the matter begins to boil is called the boiling point.
Boiling point
The boiling point of the water is 100° Celsius and this means that the water begins to boil and change into the water vapor (gas).
The pressure affects the boiling points, and the boiling points of the liquids vary widely at the normal atmospheric pressure, because of this variation in the boiling points, two or more liquids can often be separated by a process called fractional distillation.
At the boiling point, the atoms or the molecules of the liquid gain enough energy to change the liquid into water vapor, and then the boiling is rapidly evaporated.
Life application on the boiling process
The separation of the petroleum oil depends on the difference between them in their boiling points, The separation of the components of the oil can be done by heating the crude oil and then separating each substance at its boiling point.
The boiling point is the temperature at which the vapour pressure of the substance is equal to the atmospheric pressure, The boiling point depends on the pressure, whereas the pressure increases, the boiling point increases.
The pressure pans are used for fast cooking as they raise the pressure so, the boiling point increases and the food is cooked faster.
Separation of the petroleum oil
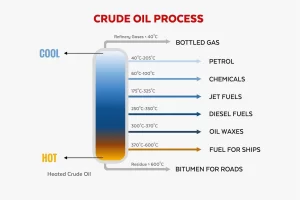
The separation of petroleum oil is also known as crude oil refining, It is a process that involves breaking down crude oil into its useful components. This process is primarily carried out through fractional distillation and other refining techniques to produce fuels and other products.
1. Desalting
Crude oil is often contaminated with water, salts, and sediments. The first step in refining is desalting, where these impurities are removed by washing the crude oil with water. This helps prevent corrosion in the refining equipment.
2. Fractional Distillation
This is the primary method used to separate crude oil into different fractions based on boiling points. Crude oil is heated to about 350-400°C (662-752°F), causing it to vaporize. The vapor enters a fractionating column, where it rises through trays or packing material.
As the vapor cools, different hydrocarbons condense at various levels of the column, depending on their boiling points. Lighter fractions condense at the top, and heavier ones condense at the bottom.
Some common fractions include:
- Petroleum gases (propane, butane) – top of the column, around 20-100°C.
- Naphtha (used for gasoline production) – 70-200°C.
- Kerosene (jet fuel) – 150-300°C.
- Diesel – 250-350°C.
- Heavy gas oils (lubricating oils, fuel oils) – 300-400°C.
- Residuum (asphalt, bitumen) – bottom of the column, remains as the heaviest fraction.
3. Conversion Processes
Some fractions, like heavy oils, may not be useful as-is. Refineries use conversion processes to break these heavy fractions into lighter, more valuable products. These processes include:
- Cracking: Large hydrocarbons are broken into smaller molecules. Types include thermal cracking (using heat) and catalytic cracking (using catalysts).
- Hydrocracking: Uses hydrogen and catalysts to break down heavier fractions into lighter, cleaner products like gasoline and diesel.
4. Treating and Reforming
- Treating removes impurities like sulfur, nitrogen, and metals from the fractions. This process uses techniques like hydrotreating or sweetening to make the products more environmentally friendly.
- Reforming converts the low-octane naphtha into high-octane gasoline by rearranging hydrocarbon molecules, It produces valuable byproducts like hydrogen.
5. Blending
The final products are blended to meet market specifications for fuels such as gasoline, diesel, and jet fuel. Additives may be mixed in to improve performance or reduce environmental impact.
6. Additional Processing Units
Coking: Used to process the heaviest residual oils, yielding lighter products and petroleum coke (used in industrial applications).
Isomerization and Alkylation: Produce higher-octane gasoline components by altering molecular structures.
Final Products
- Fuels such as Gasoline, diesel, kerosene, jet fuel, and fuel oil.
- Lubricants: Motor oils, greases.
- Petrochemicals are used to make plastics, synthetic rubber, and chemicals.
- Other byproducts: Asphalt, paraffin wax, sulfur.
This entire refining process transforms crude oil into usable products by separating it into various hydrocarbon chains with specific applications.
Offshore Drilling advantages and disadvantages
Properties of fluids, Factors affecting density and pressure
Applications on the pressure at a point (Connected vessels, U-shaped tube & Mercuric barometer)