The mole , Mass of substance & Avogadro’s number
The mole
Mole is the unit of the quality of matter in the International System of Units ( SI ) and it is defined by different ways according to the mole and mass of substance , the mole and Avogadro’s number and the mole and volume of gas .
Molecule is the smallest part of a substance that can found in a single form and the properties of the substance depend on it , The atom is the smallest building unit of the substance that can participate in the chemical reactions .
The molecule and the atom are very small particles, their dimensions are expressed in the Nano-meter unit and it is very difficult to deal with them practically.
We can not deal with limited atoms , molecules or ions due to their small masses and volumes as we use enormous numbers , where the least mass from the substance contains a huge number of atoms , molecules or ions .
The first scientist who named the mole was the scientist , Wilhelm Ostwald , in 1894 and was originated from the German word mole , It is derived from the word Molecule .
The mole and mass of substance
The mole is the atomic mass , molecular mass or mass of formula unit expressed in grams , Any substance may be found in the form of atoms , molecules or formula units , We can not say a molecule of ionic compound , but it is called formula unit .
If the substance is in the form of atoms ( the building unit of substance is atom )
The mass of one atom is called atomic mass which is very small , It differs from one atom to another one which is estimated by atomic mass unit ( amu ) or u , The molar mass of different elements are different because they have different atomic masses .
The atomic mass of an element is called molar mass of atoms which is expressed in grams ( g ) , If the atomic mass of carbon atom represents 12 g of it , So , the molar mass of carbon atoms = 12 g / mol .
If the substance is in the form of molecules ( the building unit of substance is molecule )
The mass of one molecule is the sum of the atomic masses of the atoms forming that molecule , So , Molecular mass is the sum of the atomic masses of the atoms forming the molecules , the mass of one mole of molecule is called molar mass of molecule , which is expressed in grams ( g ) .
The mass of a mole differs from one matter to another due to the difference of matters in their molecular composition , therefore they differ in their molecule mass .
There are some elements have different molecular formulas depending on their physical state , So , The mass of one mole will differ from one state to the other .
The difference between the molar mass of phosphorus from solid state to vapour state because of the difference between their molecular formula of the solid state and vapour state , according to the difference in the molecular mass .
The molar mass of oxygen molecule differs from that of oxygen atom because one mole of oxygen molecule contains two moles of oxygen atoms , at which the oxygen is a diatomic molecule .
If the substance is in the form of ionic compound ( the building unit of substance is formula unit )
The ionic compounds are found in the form of crystal lattice , as the ion is surrounded from all directions with the ions of an opposite charge , The ionic compounds can be expressed by the Formula unit , which shows the ratio between the ions forming it .
The mole and Avogadro’s number
The Italian scientist Amedeo Avogadro reached the result that the mole of any substance contains a constant number of ( atoms , molecules , formula units or ions ) , this number equals 6.022140857 × 1023 and it is called Avogadro’s number .
Avogadro’s number is the number of the atoms , the molecules , formula units or ions which are found in one mole of substance and it equals 6.02 × 1023 ( atom , molecule or ion ) .
The mole is the amount of a substance that contains Avogadro’s number of particles ( molecular , atoms , ions or formula units ) .
The number of moles
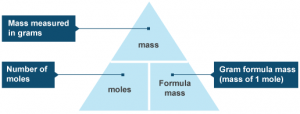
Number of moles ( mol ) = Mass of substance ( g ) ÷ Molar mass ( mass of one mole ) ( g/mol )
The number of ( molecules , atoms , formula units , ions ) = No. of moles of ( molecules , atoms , formula units , units ) Χ 6.02 × 1023
Limiting reactant of Chemical Reaction , Mole and volume of gases