Matter construction and properties of molecules of matter
The molecules
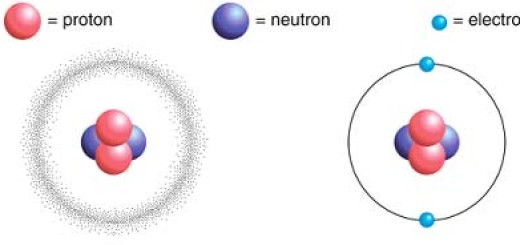
The matter consists of very small structural units known as the molecules, The molecule is the smallest part of the matter which can exist freely, and it has the properties of the matter.
The properties of the molecules of the matter
The molecules of the matter are in a continuous motion and there are intermolecular spaces and attraction forces among the molecules of the matter, The kinds of the molecules are the element, the compound and the mixture.
The intermolecular spaces are the spaces that are found among the molecules of the matter, The intermolecular force is the force that bounds the molecules of the matter together.
The molecules of one substance are alike in properties, but they differ from the other substance molecules, The molecules are composed of tiny structural units called atoms.
The difference in the molecules of the various substances is found as the result of the difference of the atoms which are involved in the structure of the molecule and depends on the number and the kind of the atoms, and the way of the combination together.
The element molecule
The element is the simplest pure form of matter which can not be analyzed chemically into the simpler form, The element molecule is formed of similar atoms (one or more atoms) that combine together.
Monoatomic elements
There are elements molecules that are formed of only one atom (The Monoatomic) such as, the solids as (Copper, Iron, Aluminum, Sulphur, Magnesium and Carbon) and the liquids as mercury.
The noble gases such as (Helium, Neon, Argon, Krypton, Xenon and Radon).
Diatomic elements
There are elements molecules that formed of two atoms (Diatomic) such as
The liquids as bromine.
The active gases such as (Oxygen, Hydrogen, Chlorine, Nitrogen and Fluorine).
 |
| Oxygen molecule is an active gas, and the molecules of the active gases are diatomic. |
The compound molecule
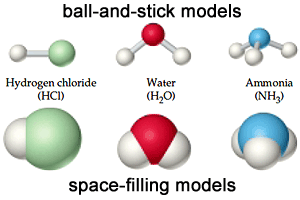 |
| The compound molecule is formed from different atoms. |
The compound molecule is formed of different atoms and it is a substance that is formed from a combination of the atoms of two or more different elements with constant weight ratios.
Examples of compound molecules:
Sodium chloride molecule (table salt), Water molecule, Ammonia molecule, Hydrogen chloride.
You can download Science online application on google play from this link: Science online Apps on Google play
Matter, Properties & Kinds of molecules, Melting process & Vaporization process
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept