Colorimetry, Colorimeter components and Estimation of Hemoglobin concentration by Colorimetry
Many important biological solutions are estimated as coloured solutions, in which the intensity of the colour is proportionate to the concentration of the biological substance. When light passes in a coloured solution, it has a specific wavelength, therefore, the intensity of the colour is measured as its optical density or absorbance of its wavelength.
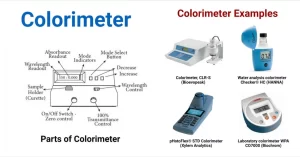
Colorimetry
Colorimeter is a device that measures the absorbance or optical density or optical activity of the coloured solutions, or absorbance of their wavelengths, and the intensity of colour is read on its scale. The sample (test) with an unknown concentration is compared with a standard solution of well-known concentration to obtain the exact concentration of that sample.
The following formula is usually used:
- Conc. sample = (A sample/A standard) X Conc. Standard
- A sample = Absorbance (optical density, reading) of the sample.
- A standard = Absorbance (optical density, reading) of the standard.
- Conc. sample = Concentration of the sample (test).
- Conc. Standard = Concentration of the known standard solution.
The zero point of the colorimeter should be first adjusted with a solution known as the blank, A water blank (colourless solution) or a reagent blank may be used, Sometimes the zero point of the colorimeter is adjusted against air, and this depends upon the procedure used.
Colorimeter
A colorimeter is a device to measure the intensity of light after it has passed through an absorbing medium, In its simplest form, it consists of a light source, Lenses, Filter, Cuvette, Photocell, and Galvanometer.
Components of Colorimeter
- Light Source: A low level of illumination (usually from 6-12 V lamp) is used.
- Condenser lenses: They give parallel rays.
- Filter: A filter consists of a material that selectively transmits the desired wavelength and absorbs all other wavelengths, Its colour is complementary to that of coloured solution to allow maximum absorption and transmission.
- Cuvette: Cuvettes are optically transparent cells used to contain the “coloured” solution under study. In general, the length, the length of a light path across the cuvette is usually kept constant at 1 cm, Scratched or contaminated cuvettes result in inaccurate work.
- Photocell: A photocell measures the intensity of the light transmitted through the solution after absorption, It converts quanta of radiation to electrical energy which may be amplified, detected and recorded, Thus, the photocell has the function of generating an electric current proportional to the light falling on it from the coloured solution according to the intensity of its colour.
- Galvanometer: It contains the scale of reading, The output (electrical current) of the photocell can now be read by a sensitive meter system, The reading is proportional to the intensity of the electric current emitted from the photocell and consequently to the intensity of colour of the solution of the test sample of unknown conc. under investigation.
Application:
- Colorimetry helps in measuring the concentration of any substance, If the substance by itself is colourless, it can be chemically converted into a coloured substance.
- The colorimeter can also be used to measure the turbidity of solutions (e.g. bacterial suspension), but the nephelometer is a better measure of turbidity.
A spectrophotometer is a much more refined version of a colorimeter, In a colorimeter, filters are used which allow a broad range of wavelengths to pass through, whereas in the spectrophotometer a prism is used to split the incident beam into different wavelengths, By specific mechanisms, waves of specific wavelengths can be manipulated to fall on the test solution, The spectrophotometer is useful for measuring the absorption spectrum of a compound, that is, the absorption of light by a solution at each wavelength.
Estimation of Hemoglobin concentration by colorimetry
Hemoglobin is a protein with a prosthetic group haem united to the protein globulin. Its molecular weight is 68000. Haem is an iron-prophyrin compound. Porphyrin is formed of four pyrrole rings united together by four methane bridges.
Normal Value
- Adult male: 14-18 g/dl blood.
- Adult female: 12-16 g/dl blood.
When hemoglobin level decreases below the normal value, this denotes the presence of anaemia, while an increase in its level is found in polycythemia.
Determination of hemoglobin concentration in blood
Principle: Hemoglobin is oxidized by potassium ferricyanide into methaemoglobin which is converted into cyanomethemoglobin by potassium cyanide. The intensity of absorbance of cyanomethaemoglobin is proportional to hemoglobin concentration.
Reagents
1- Ferricyanide-cyanide reagent (Haemoglobin solution):
- 200 mg potassium ferricyanide.
- 50 mg potassium cyanide.
- 140 mg potassium dihydrogen phosphate.
- Dissolve all reagents in one litre distilled water and keep in bottle at 5- 25° C.
2- Cyanomethaemoglobin standard: 15 g/dl.
Examination of haemoglobin and its derivatives
Haemoglobin Derivatives
1- Oxyhaemoglobin (HbO2)
A) Preparation:
- Stir well the defibrinated blood before use, because the red corpuscles possess a high specific gravity and tend to settle down.
- Dilute in a cylinder 1 ml of blood up to 100 ml with water, and mix well. You get now a diluted sample of blood with a dilution of 1:100.
- Fill about half of a test tube with the diluted blood, notice its colour and examine by the spectroscope.
B) Colour of the sample: Bright red (like the colour of arterial blood).
2- Haemoglobin (Reduced haemoglobin, Hb):
A) Preparation
- Fill about half of a test tube with the diluted blood prepared in the previous experiment.
- Add a few drops of a concentrated solution of a reducing agent e.g. sodium dithionite (or sodium hyposulphite).
- Notice the change of colour of the sample, and then examine it by the spectroscope.
B) Colour of the sample: Red with a bluish tinge (like the colour of venous blood).
To differentiate between HbO2 and HbCO (caused by carbon monoxide poisoning and causing cherry red colour of blood) in a blood sample, add a few drops of sodium dithionite to the sample, if it is HbO2, it will be reduced and colour will change to bluish like reduced hemoglobin. If the sample is HbCO, no reduction will occur and the colour won’t change. The affinity of haemoglobin to combine with CO is 200 times more than that of O2.
3. Met haemoglobin (Met-Hb, HbO):
A) Preparation:
- Prepare a 1:20 diluted solution of blood.
- Place 5 ml of the diluted blood (1:20) in a test tube and add a few drops of an oxidizing agent eg. 5 drops of freshly prepared 10% solution of potassium ferricyanide, then mix.
- Notice the change of colour of the prepared sample.
B) Colour of the sample: Brown-red
- The divalent Fe2+ in HbO2 is oxidized to the trivalent Fe3+ in met-Hb.
- Met-Hb can be also prepared by treating blood with various oxidizing agents such as nitrites, permanganate, nitrobenzene, etc.
- Methemoglobinmeia can be either congenital or acquired.
Congenital causes include a mutation where histidine is replaced by tyrosine or a deficiency in the cytochrome b5 methemoglobin reductase enzyme system. Acquired causes include people living near factories and are subjected to oxidizing agents leading to the formation of more met- Hb in their blood and consequently their respiratory capacity is reduced, in addition to some drugs like nitrates that increase met-Hb.
Treatment
In case of mutation, the only treatment is blood transfusion, while in case of deficiency in reducing enzyme system or acquired causes, such people can be treated by taking reducing agents e.g. vitamin C and glucose.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download the Science Online application on Google Play from this link: Science Online Apps on Google Play
Blood analysis, Hemolysis, importance, structure, types and hemolysis test results
Cloning of DNA sequences, Importance of recombinant DNA & Uses of human genome
Molecular technology (Genetic Engineering) advantages, Techniques and uses
Importance of Nucleosides, Nucleotides, Purines, Pyrimidines & Sugars of nucleic acids
Regulation of the cell cycle, DNA synthesis phase, Interphase & Mitosis