Classification of Acids according to its strength (degree of ionization), Its source & Basicity
Acids can be classified according to its strength ( degree of ionization ) , Its source and Basicity , Acids are classified according to its degree of ionization in the aqueous solution into Strong acids and weak acids .
Classification of acids according to its strength ( degree of ionization )
Strong acids
The acids which are completely ionized in the water , Their aqueous solution conducts the electric current to a large degree, They are considered as strong electrolytes .
Examples : Hydroiodic acid HI, Hydrochloric acid HCl, Hydrobromic acid HBr, Sulphuric acid H2SO4, Nitric acid HNO3 & Perchloric acid HClO4 .
Weak acids
The acids which are incompletely ionized in the water , Their aqueous solution conducts the electric current to a small degree, They are considered as weak electrolytes .
Examples : Carbonic acid H2CO3 , Phosphoric acid H3PO4 , Acetic acid ( vinegar ) CH3COOH , Formic acid , Citric acid , Oxalic acid & Lactic acid .
There is no relation between the strength of the acid and the number of the hydrogen atoms in its molecular structure , Phosphoric acid ( H3PO4 ) is weaker than nitric acid ( HNO3 ) , although it contains greater number of hydrogen atoms .
CH3COOH ( aq ) + H2 O ( l ) ↔ CH3COO− ( aq ) + H3 O+ (aq)
Hydrochloric acid HCl is stronger than acetic acid because HCl is completely ionized in the water , but acetic acid is partially ionized in the water .
HCl ( aq ) + H2 O ( l ) → Cl− ( aq ) + H3 O+ (aq)
Classification of acids according to their sources
Acids are classified according to their sources ( origin ) into Organic acids and Mineral acids
Organic acids
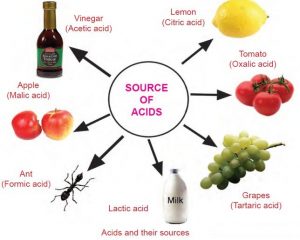
Acids that have an organic origin ( plant or animal ) , They are extracted from the organs of living organisms , All of them are weak acids .
Examples : Lactic acid ( milk products ) , Acetic acid ( vinegar ) , Citric acid ( from lemon ) , Oxalic acid and Formic acid .
Mineral acids
Acids that have no organic origin , They usually have non-metallic elements in their structure like chlorine , sulphur , nitrogen and phosphorus , Some of them are strong acids and others are weak .
Examples : Carbonic acid H2CO3 , Hydrochloric acid HCl , Phosphoric acid H3PO4 , Perchloric acid HClO4 , Nitric acid HNO3 & Sulphuric acid H2SO4 .
Classification of acids according to basicity
The basicity of acid
It is the number of hydrogen ions ( H+ ) , which is produced by one molecule of the acid when it dissolves in the water .
Acids are classified according to the basicity into Monobasic acids ( Monoprotic ) , Dibasic acids ( Diprotic ) and Tribasic acids ( Triprotic ) .
Monobasic acids ( Monoprotic )
Acids where each molecule gives one proton H+ , when it dissolves in the water .
Examples : Organic monobasic acids ( Formic acid HCOOH , Acetic acid CH3COOH ) , Mineral monobasic acids ( Hydrochloric acid HCl , Nitric acid HNO3 ) .
Dibasic acids ( Diprotic )
Acids where each molecule gives one or two protons H+, when it dissolves in the water .
Examples : Organic diabasic acids ( Oxalic acid ) , Mineral dibasic acids ( Sulphuric acid H2SO4 , Carbonic acid H2CO3 ) .
Tribasic acids ( Triprotic )
Acids where each molecule gives one, two or three protons H+, when it dissolves in the water .
Examples : Organic tribasic acids ( Citric acid ) , Mineral tribasic acids ( Phosphoric acid H3PO4 ) .
Both of citric acid and phosphoric acid have the same degree of basicity, while they differ in their source ( origin), Because both of them are tribasic acids , but citric acid is an organic acid, while phosphoric acid is a mineral acid.
Classifications of bases according to strength ( degree of ionization ) and molecular structure
Properties of Acids and Bases & Theories defining acids and bases
Types of compounds, Properties of Acids, Bases (alkalis), Oxides and Salts